
���ܵĴ洢������Ӧ�õ���Ҫƿ������λ�⻯��������廯������Ŀǰ�����õ���Ҫ������ϡ�
(1)Ti(BH4)2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ��ڻ�̬Ti2���У�����ռ�ݵ�����ܲ����Ϊ________�����ܲ���е�ԭ�ӹ����Ϊ______��
(2)Һ���Ǹ������ʣ������ܵ��������壬����N2��3H22NH3ʵ�ִ�������⡣����˵����ȷ����________________________________________________________________________��
a��NH3�����е�ԭ�ӵĹ���ӻ���ʽΪsp2�ӻ�
b��NH ��PH
��PH ��CH4��BH
��CH4��BH ��ClO
��ClO ��Ϊ�ȵ�����
��Ϊ�ȵ�����
c����ͬѹǿʱ��NH3�ķе��PH3�ķе��
d��[Cu(NH3)4]2����Nԭ������λԭ��
(3)��֪NF3��NH3�Ŀռ乹����ͬ����NF3������Cu2���γ������ӣ���ԭ����________________________________________________________________________
________________________________________________________________________��
��.�Ȼ����������еij��õ�ζƷ��Ҳ�ǽṹ��ѧ���о����Ӿ���ʱ���õĴ�����侧���ṹ��ͼG133��ʾ��

ͼG133
(1)���Ȼ��ƾ�����Na�����������ڵ�Cl��֮��ľ���Ϊr�����Na��������ν��ڵ�Cl���ĸ���Ϊ______����Na��������ν��ڵ�Cl��֮��ľ���Ϊ______��
(2)��֪���Ȼ��ƾ�����Na���İ뾶Ϊa pm��Cl���İ뾶Ϊb pm�������ھ������ǽ��ܽӴ��ģ������Ȼ��ƾ��������ӵĿռ�������Ϊ________________________________________________________________________
(�ú�a��b��ʽ�ӱ�ʾ)��
(3)���ײ��ϵı���ԭ��ռ��ԭ�����ı����ܴ��������������������ʵ�ԭ����ij�Ȼ��ƿ�����״Ϊ�����壬�߳�Ϊ�Ȼ��ƾ�����10��������Ȼ��ƿ����б���ԭ��ռ��ԭ�����İٷֱ�Ϊ________________��
��.(1)M��9��(2)cd
(3)N��F��H����Ԫ�صĵ縺�Դ�СΪF>N>H����NF3�У����õ��Ӷ�ƫ��Fԭ�ӣ�ƫ��Nԭ�ӣ�ʹ�õ�ԭ���ϵŵ��Ӷ�������Cu2���γ���λ��
��.(1)8��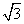 r��(2)
r��(2)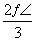 ��
��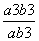 ��100%
��100%
(3)26%��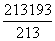 ��100%
��100%
[����] ��.(1)����22��Ԫ�أ�Ti2���ĺ�����20�����ӣ����ݹ���ԭ��֪���������Ų�ʽΪ1s22s22p63s23p63d2��ռ����������ĵ��Ӳ�ΪM�㡣���ܲ������ӵĹ����3s��3p��3d��9�������
(2)NH3������Nԭ�Ӻ���3�����õ��ӶԺ�1���µ��Ӷԣ�������۲���Ӷ�����4��Nԭ�Ӳ���sp3�ӻ���a����ClO ��NH
��NH ���ǵȵ����壬b������ͬѹǿʱ�����������к��������PH3�в������������NH3�ķе��PH3�ߣ�c��ȷ��[Cu(NH3)4]2����Nԭ���ṩ�µ��Ӷԣ�����Nԭ������λԭ�ӣ�d��ȷ��
���ǵȵ����壬b������ͬѹǿʱ�����������к��������PH3�в������������NH3�ķе��PH3�ߣ�c��ȷ��[Cu(NH3)4]2����Nԭ���ṩ�µ��Ӷԣ�����Nԭ������λԭ�ӣ�d��ȷ��
��.(1)��Na���ν��ڵ�Cl�����ھ���������λ�ã��ʴν��ڵ�Cl����8���������֮��ľ���Ϊx����x2��r2��2r2��x��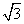 r��
r��
(2)1�������к���Na����Cl����Ϊ4����1��Na����1��Cl���������Ϊ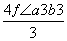 �������������Ϊ(2a��2b)3��������Ŀռ�������Ϊ
�������������Ϊ(2a��2b)3��������Ŀռ�������Ϊ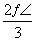 ��
��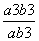 ��100%��
��100%��
(3)�����������е���ԭ����Ϊn3(nΪ����ԭ����)����˱߳�Ϊ�Ȼ��ƾ����߳���10�����Ȼ��ƿ�������ԭ����Ϊ213�������ڲ�����ԭ����Ϊ193(�൱�ڽ��������һ��)������������ԭ����Ϊ213��193������ԭ��ռ��ԭ�����İٷ�����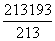 ��100%��26%��
��100%��26%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
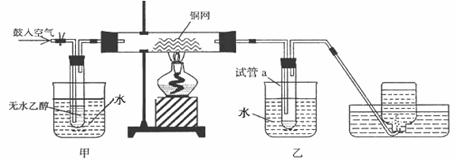
(1)��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�
˵�����Ҵ�����Ӧ��_____ ��Ӧ��
(2)����������ˮԡ���ò���ͬ��
��������____________�� �ҵ�������_______________��
(3)����Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�
������ __��
����ƿ���ռ������������Ҫ�ɷ���____________ ��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________________ ��
Ҫ��ȥ�����ʣ����ڻ��Һ�м���_____________����д��ĸ����
�ᣮ�Ȼ�����Һ����b������ c��̼��������Һ������d�����Ȼ�̼
Ȼ����ͨ��___________________����ʵ��������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ��ˮ�г�����һ������Cr2O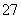 ��CrO
��CrO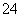 �����ǻ�����༰��̬ϵͳ�����ܴ���������д��������õĴ������������֡�
�����ǻ�����༰��̬ϵͳ�����ܴ���������д��������õĴ������������֡�
����1����ԭ������
�÷��Ĺ�������Ϊ
CrO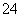
 Cr2O
Cr2O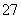
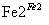 Cr3��
Cr3��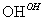 Cr(OH)3��
Cr(OH)3��
���еڢٲ�����ƽ�⣺
2CrO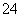 (��ɫ)��2H��
(��ɫ)��2H�� Cr2O
Cr2O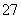 (��ɫ)��H2O
(��ɫ)��H2O
(1)��ƽ����ϵ�� pH �� 2������Һ��________ɫ��
(2)��˵���ڢٲ���Ӧ��ƽ��״̬����________��
a��Cr2O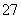 ��CrO
��CrO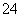 ��Ũ����ͬ
��Ũ����ͬ
b��2v(Cr2O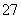 )��v(CrO
)��v(CrO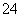 )
)
c����Һ����ɫ����
(3)�ڢڲ��У���ԭ 1 mol Cr2O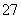 ���ӣ���Ҫ________mol��FeSO4��7H2O��
���ӣ���Ҫ________mol��FeSO4��7H2O��
(4)�� �۲����ɵ�Cr(OH)3 ����Һ�д������³����ܽ�ƽ�⣺
Cr(OH)3(s)  Cr3��(aq)��3OH��(aq)
Cr3��(aq)��3OH��(aq)
�����£�Cr(OH)3 ���ܶȻ�Ksp��c(Cr3��)��c3(OH��)��10��32��Ҫʹ c(Cr3��)����10��5mol/L����Һ��pHӦ����________��
���ܶȻ�Ksp��c(Cr3��)��c3(OH��)��10��32��Ҫʹ c(Cr3��)����10��5mol/L����Һ��pHӦ����________��
����2����ⷨ
�÷��� Fe ���缫��⺬Cr2O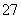 �����Է�ˮ�����ŵ��Ľ��У�������������Һ pH ���ߣ����� Cr(OH)3 ������
�����Է�ˮ�����ŵ��Ľ��У�������������Һ pH ���ߣ����� Cr(OH)3 ������
(5)��Fe���缫��ԭ��Ϊ_____________________ _______________________��
_______________________��
(6)������������Һ pH ���ߵ�ԭ����(�õ缫��Ӧ����)________����Һ��ͬʱ���ɵij�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڷ��ֵ�һ����Ȼ��ʮ������������Al��Cu��Fe���ֽ���Ԫ����ɡ��ش��������⣺
(1)����һ����ƽ�����������ϸ�����λ����Ķ��ؾ��壬��ͨ��________�������־��塢����ͷǾ��塣
(2)��̬Feԭ����________��δ�ɶԵ��ӣ�Fe3���ĵ����Ų�ʽΪ______________________________���������軯�ؼ���Fe3�����γɵ���������ɫΪ________��
(3)���Ʊ���Cu(OH)2�ɽ���ȩ(CH3CHO)���������ᣬ��������ԭ��Cu2O����ȩ��̼ԭ�ӵ��ӻ��������Ϊ______________��1 mol��ȩ�����к��еĦҼ�����ĿΪ____________������ķе����Ը�����ȩ������Ҫԭ����__________________________________��Cu2OΪ�뵼����ϣ��������������ڲ���4����ԭ�ӣ�������ԭ��λ�����ĺͶ��㣬��þ�������________��ͭԭ�ӡ�
(4)Al����Ϊ�����������壬�侧������a��0.405 nm����������ԭ�ӵ���λ��Ϊ________����ʽ��ʾAl���ʵ��ܶ�________________________________________________________________________g��cm��3
(���ؼ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£� Ni2���붡��ͪ������ʺ�ɫ����A��
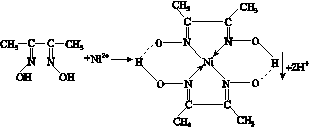
������������������������������A
(1)��̬Ni2���ĺ�������Ų�ʽΪ________________________________________________________________________��
(2)����ͪ뿵����Ԫ����C��N��O�ĵ縺���ɴ�С��˳��Ϊ________������ͪ뿷�����Cԭ�ӵ��ӻ����������________��
(3)Ԫ��Ni��һ����(Te)���ᄃ��ľ����ṹ��ͼK333��ʾ����û�����Ļ�ѧʽΪ________��

ͼK333
(4)Ni(CO)4��һ����ɫҺ�壬�е�Ϊ42.1�棬�۵�Ϊ��19.3 �档Ni(CO)4�ľ���������________����д��1���ɵڶ���������Ԫ����ɵ�����CO��Ϊ�ȵ�����������ӵĵ���ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���� �� ��
A.40K�� 40Caԭ�������������������������
B.���ʯ��ʯī��������ͬ
C.H2��D2��Ϊͬλ��
D.ij����ֻ��һ��Ԫ�أ�������һ���Ǵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У���һ�������¼�����ӳɷ�Ӧ��Ҳ����ȡ����Ӧ��������ʹKMnO4������Һ��ɫ����
A������ B���� C����ϩ D���Ҵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ǻ�ѧѧϰ�о����õ��ķ��������ж�һЩ���ʻ�ѧ��Ӧ�ķ����Լ�������ȷ����
A��H2SO4���ᣬ��ΪH2SO4�к�����Ԫ��
B�������ǽ��壬��Ϊ�����еķ�ɢ������ֱ����1��100 nm֮��
C��Na��H2O�ķ�Ӧ�������ӷ�Ӧ����Ϊ��Ӧ��û�����Ӳμ�
D��Na�� Cl2��ȼ�ղ���������ԭ��Ӧ����Ϊû�е�����ʧ��
Cl2��ȼ�ղ���������ԭ��Ӧ����Ϊû�е�����ʧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ϲ��ᳫ����Ũ���ˣ���Ϊ��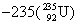 ������ԭ�ӵ���ȼ�ϡ�
������ԭ�ӵ���ȼ�ϡ�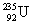 ԭ�Ӻ��ں���������Ϊ
ԭ�Ӻ��ں���������Ϊ
A��92 B��235 C��143 D��327
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com