

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
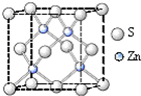 Ga��As��һ�������¿��Ժϳ�GaAs��GaAs��һ�����ͻ�����뵼����ϣ������ܱȹ����Խ����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȣ�
Ga��As��һ�������¿��Ժϳ�GaAs��GaAs��һ�����ͻ�����뵼����ϣ������ܱȹ����Խ����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȣ�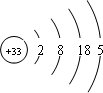

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������ɽ���������һ��ѧ���£� ���ͣ�013
Se�ǰ뵼����ϣ�Ҳ�Dz�������Ʒ�е�Ԫ�أ���ҵ����ȡ���ķ���֮һ����H2SO4��NaNO3������Se�Ĺ�ҵ���϶��õ�������(H2SeO3)����������(H2SeO4)�����������Ṳ�ȿ����������ᣬ��ӦΪH2SeO4��2HCl��H2SeO3��Cl2����H2O����SO2ͨ����������Һ���е������������ݴˣ�����˵������ȷ����
[����]
A��H2SeO4������ǿ��Cl2
B��H2SeO3������ǿ��ϡH2SO4
C��SeO2�Ļ�ԭ��ǿ��SO2
D������1mol Se��H2SeO3��SO2��H2O��1mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com