
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |

 HCO3-+OH-���ʴ�Ϊ��CO32-+H2O
HCO3-+OH-���ʴ�Ϊ��CO32-+H2O HCO3-+OH-��
HCO3-+OH-��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ���� | 1 | 2 | 3 |
| ����Na2S2O3���/mL | 26.90 | 27.00 | 26.96 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2009?�ع�һģ��ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
��2009?�ع�һģ��ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮 HCO3-+OH-
HCO3-+OH- HCO3-+OH-
HCO3-+OH-| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�λ���ѧ2011������������(��)��ѧ���� ���ͣ�058
ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
(1)��pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ�________��
(2)����ǿ������õ�pHС��7�������ɣ�________��
������������NaOH��Һ�еμӼ��η�̪��Һ����Һ�Ժ�ɫ��Ȼ���ٵμ����ᣬ�ɹ۲쵽��ɫ����ʧ����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
����ͬѧ����NaOH��Һ�еμӷ�̪��Һʱ��������һ��������������������Һ�е����̪��Һ����Һ����˺�ɫ������һ�����ɫ����ʧ�ˣ���С����������������ԭ���������²��룺
�ٿ����Ƿ�̪��Һ������е�������Ӧ��ʹ��ɫ��ʧ��
�ڿ���������������Һ������еĶ�����̼��Ӧ��ʹ��ɫ��ʧ��
(1)Ϊ��֤����٣�����ͬѧ�����Ƶ�����������Һ���ȣ�����Һ���Ϸ���һЩֲ���ͣ�Ȼ������ȴ�����Һ�е����̪��Һ��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ����________��ʵ����������̪��Һ��ɫ��ʧ������е������أ�
(2)Ϊ��֤����ڣ�����ͬѧ��������ʵ�飺ȡ��һ������Na2CO3��Һ�������е����̪��Һ��������ҺҲ���ֺ�ɫ���������ӷ���ʽ������һ���������ԭ��________�ɴ�˵����̪��Һ��ɫ��ʧ������еĶ�����̼�أ�
(3)��С��ͬѧͨ���������ϵ�֪��������������ҺŨ�ȴ���2 mol/Lʱ���ͻ���������������������ʵ��֤���÷�����ȡ�õ�NaOH��ҺŨ�ȹ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��14�֣�ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�顣
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ� ��
��2������ǿ������õ�pHС��7�������ɣ� ��
������������NaOH��Һ�еμӼ��η�̪��Һ����Һ�Ժ�ɫ��Ȼ���ٵμ����ᣬ�ɹ۲쵽��ɫ����ʧ����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
����ͬѧ����NaOH��Һ�еμӷ�̪��Һʱ��������һ��������������������Һ�е����̪��Һ����Һ����˺�ɫ������һ�����ɫ����ʧ�ˡ���С����������������ԭ���������²��룺
�ٿ����Ƿ�̪��Һ������е�������Ӧ��ʹ��ɫ��ʧ��
�ڿ���������������Һ������еĶ�����̼��Ӧ��ʹ��ɫ��ʧ��
��1��Ϊ��֤����٣�����ͬѧ�����Ƶ�����������Һ���ȣ�����Һ���Ϸ���һЩֲ���ͣ�Ȼ������ȴ�����Һ�е����̪��Һ��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ���� ��ʵ����������̪��Һ��ɫ��ʧ������е������ء�
��2��Ϊ��֤����ڣ�����ͬѧ��������ʵ�飺ȡ��һ������Na2CO3��Һ�������е����̪��Һ��������ҺҲ���ֺ�ɫ���������ӷ���ʽ������һ���������ԭ��
��
�ɴ�˵����̪��Һ��ɫ��ʧ������еĶ�����̼�ء�
��3����С��ͬѧͨ���������ϵ�֪��������������ҺŨ�ȴ���2mol/Lʱ���ͻ���������������������ʵ��֤���÷�����ȡ�õ�NaOH��ҺŨ�ȹ���
��
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10 mL��ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t�����±�����
��� | ���� | NaOH��Һ | ��t/�� |
1 | 3.65 | 2.00 | 3.5 |
2 | 3.65 | 4.00 | x |
3 | 7.30 | 8.00 | 14 |

��1������x = ��
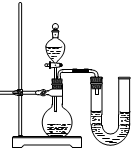
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�顣���ͬѧ���� ������������������������ ��������ʵ�������ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com