
��14�֣�ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH4+��6HCHO===3H����6H2O��(CH2)6N4H��[�ζ�ʱ��1 mol(CH2)6N4H����1 mol H���൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
�����ȡ��Ʒ1.5 g��
�������Ʒ�ܽ���250 mL����ƿ�У����ݣ����ҡ�ȡ�
�������ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У�����10 mL 20%�����Լ�ȩ��Һ��ҡ�ȡ�����5 min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)��������������Ƿ���ȷ________(��ǡ���)��������ȷ�������_______________________________________________��(����ȷ���˿ղ���)
(2)���ݲ������գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����________(�ƫ����ƫС������Ӱ�족)��
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�________��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ����Һ���ձ��____________ɫ��
(3)�ζ�������±���ʾ��
| �ζ� ���� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�ŷ���Ʒ�ܽ���С�ձ��У���ȴ��ת��������ƿ�С�
�Ƣ�ƫ�� ����Ӱ�� ��B �ܺ�ɫ ��18.85��
���������������1������ƿ����ֱ���ܽ�����������Һ�����Բ�������Ӧ�ý���Ʒ�ܽ���С�ձ��У���ȴ��ת��������ƿ�С�
(2)�ټ�ʽ�ζ���������ˮϴ�Ӻ���Ҫ����NaOH��Һ��ϴ�������൱��NaOH��Һ��ϡ�ͣ��ζ����ĵ������ƫ�ߣ������Ʒ�е�����������Ҳ��ƫ�ߡ� ����ƿ������ˮϴ�Ӻ���Ȼˮδ������������Һ�е�H+ �����ʵ������䣬��ζ�ʱ����NaOH����Һ�е��������Ƶ����ʵ����Ͳ��䣬Ҳ������Ӱ�졣��ע��۲���ɫ�仯��ȷ���ζ��յ㡣 �ܴ���ҺΪ���ԣ���̪ӦΪ��ɫ������ҺתΪ����ʱ����Һ��ɫ��Ϊ�ۺ�(��dz��)��
(3)����Һ�����Ӧȡ����ʵ���ƽ��ֵ��
����ȷ���ζ�ʱ���õ�NaOH����ҺΪ��20.01+19.99+20.00��/3=20.00mL
�����������Լ�ȩ��Һһ���ǹ����ģ�����1.500g ��� ���ܽ��ȡ������1/10���еζ�����0.15g���ζ��������Һ�к���H+����(CH2)6N4H+����0.02´0.1010=0.00202mol
���ݷ�Ӧʽ��ÿ����4molH+����(CH2)6N4H+��,������NH4+4mol
���Թ�����NH4+0.00202mol
���к���Ԫ��0.00202´14=0.02828g
���Ե�����������Ϊ0.02828/0.15´100%=18.85%
���㣺�к͵ζ�ʵ��
���������⿼�����к͵ζ�ʵ���ԭ���Լ��������裨������ϴ�ӡ���©����ϴ��עҺ����Һ�������� �ڵζ�����Һ����Һ�����μ�ָʾ�����ζ��յ���������ظ�2��3�Ρ����㣨ȡƽ��ֵ����


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ζ� ���� |
������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.04 |
| 2 | 25.00 | 2.00 | 22.00 |
| 3 | 25.00 | 0.20 | 20.21 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ� ����] |
������Һ�����/mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�ص���ѧ�߶�4���¿���ѧ�Ծ����������� ���ͣ�ʵ����
ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH��6HCHO===3H����6H2O��(CH2)6N4H��[�ζ�ʱ��1mol(CH2)6N4H����1molH���൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
�����ȡ��Ʒ1.500g��
�������Ʒ�ܽ����ȫת�Ƶ�250mL����ƿ�У����ݣ����ҡ�ȡ�
�������ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У�����10mL20%�����Լ�ȩ��Һ��ҡ�ȡ�����5min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)���ݲ������գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����________(�ƫ����ƫС������Ӱ�족)��
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�________��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ���ж��� ��
(2)�ζ�������±���ʾ��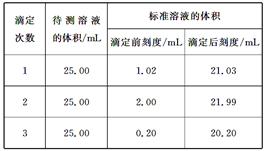
��NaOH����Һ��Ũ��Ϊ0.1010mol/L�������Ʒ�е�����������Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com