
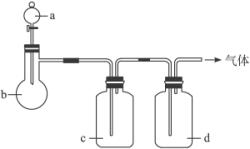
ĘųĢå | A | b | c | d |
C2H4 | ŅŅ“¼ | ÅØH2SO4 | NaOHČÜŅŗ | ÅØH2SO4 |
Cl2 | ÅØŃĪĖį | MnO2 | NaOHČÜŅŗ | ÅØH2SO4 |
NH3 | ±„ŗĶNH4ClČÜŅŗ | ĻūŹÆ»Ņ | H2O | ¹ĢĢåNaOH |
NO | Ļ”HNO3 | ĶŠ¼ | H2O | P2O5 |
£Ø1£©ÉĻŹö·½·ØÖŠæÉŅŌµĆµ½øÉŌļ”¢“æ¾»ĘųĢåµÄŹĒ______________________”£
£Ø2£©Öø³ö²»ÄÜÓĆÉĻŹö·½·ØÖĘČ”µÄĘųĢ壬²¢ĖµĆ÷ĄķÓÉ£ØæÉŅŌ²»ĢīĀś£©£ŗ
¢ŁĘųĢå________£¬ĄķÓÉŹĒ________________________________________________”£
¢ŚĘųĢå________£¬ĄķÓÉŹĒ________________________________________________”£
¢ŪĘųĢå________£¬ĄķÓÉŹĒ________________________________________________”£
¢ÜĘųĢå________£¬ĄķÓÉŹĒ________________________________________________”£
£Ø1£©NO
£Ø2£©¢ŁC2H4 ×°ÖĆ֊ƻӊĪĀ¶Č¼Ę£¬ĪŽ·ØæŲÖĘ·“Ó¦ĪĀ¶Č
¢ŚCl2 ·“Ӧɜ³ÉµÄCl2±»cÖŠµÄ NaOHČÜŅŗĪüŹÕĮĖ
¢ŪNH3 ·“Ӧɜ³ÉµÄNH3±»cÖŠµÄH2OĪüŹÕĮĖ
½āĪö£ŗĖłøųÖʱø×°ÖĆ֊ƻӊĪĀ¶Č¼Ę£¬ĪŽ·ØæŲÖĘ»ģŗĻČÜŅŗµÄĪĀ¶Č£¬²»ÄܵƵ½øÉŌļ“æ¾»µÄC2H4£»Ėłøų×°ÖĆĖäČ»¼ÓČČŹ±æÉŅŌ²śÉśCl2£¬µ«ŌŚc×°ÖĆÖŠÓĆNaOHČÜŅŗ³żHClµÄĶ¬Ź±£¬Cl2Ņ²±»ĪüŹÕ£¬×īŗó²»ÄܵƵ½Cl2£»±„ŗĶNH4ClČÜŅŗÓėCa(OH)2×÷ÓĆ²śÉśNH3£¬ŌŚNH3Ķعżc×°ÖĆŹ±±»Ė®ĪüŹÕ£¬×īŗóŅ²²»ÄÜŹÕ¼Æµ½ĘųĢ唣CuŠ¼ÓėĻ”HNO3×÷ÓĆ²śÉśNOĘųĢ壬²æ·ÖNO±»×°ÖĆÖŠµÄŃõĘųŃõ»ÆĪŖNO2£¬ŌŚĶعżcÖŠµÄĖ®Ź±£¬NO2Ņņ·¢Éś·“Ó¦3NO2+H2O====2HNO3+NOÓֵƵ½NO£¬¾P2O5øÉŌļæɵƵ½“æ¾»µÄNO”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄāÓĆĻĀĶ¼×°ÖĆÖĘČ”±ķÖŠµÄĖÄÖÖøÉŌļ”¢“æ¾»µÄĘųĢå£ØĶ¼ÖŠĢś¼ÜĢØ”¢Ģś¼Š”¢¼ÓČČ¼°ĘųĢåŹÕ¼Æ×°ÖĆ¾łŅŃĀŌČ„£»±ŲŅŖŹ±æÉŅŌ¼ÓČČ£»a”¢b”¢c”¢d±ķŹ¾ĻąÓ¦ŅĒĘ÷ÖŠ¼ÓČėµÄŹŌ¼Į£©”£
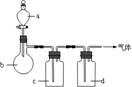
| ĘųĢå | a | b | c | d |
| C2H4 | ŅŅ“¼ | ÅØH2SO4 | NaOHČÜŅŗ | ÅØH2SO4 |
| Cl2 | ÅØŃĪĖį | MnO2 | NaOHČÜŅŗ | ÅØH2SO4 |
| NH3 | ±„ŗĶNH4ClČÜŅŗ | ĻūŹÆ»Ņ | H2O | ¹ĢĢåNaOH |
| NO | Ļ”HNO3 | ĶŠ¼ | H2O | P2O5 |
¢ÅÉĻŹö·½·ØÖŠæÉŅŌµĆµ½øÉŌļ”¢“æ¾»µÄĘųĢåŹĒ ”£
¢ĘÖø³ö²»ÄÜÓĆÉĻŹö·½·ØÖĘČ”µÄĘųĢ壬²¢ĖµĆ÷ĄķÓÉ£ØæÉŅŌ²»ĢīĀś£©
¢ŁĘųĢå £¬ĄķÓÉŹĒ ”£
¢ŚĘųĢå £¬ĄķÓÉŹĒ ”£
¢ŪĘųĢå £¬ĄķÓÉŹĒ ”£
¢ÜĘųĢå £¬ĄķÓÉŹĒ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”·šÉ½Ņ»ÖŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©ÄāÓĆĻĀĶ¼×°ÖĆÖĘČ”±ķÖŠµÄĖÄÖÖøÉŌļ”¢“æ¾»µÄĘųĢå£ØĶ¼ÖŠĢś¼ÜĢØ”¢Ģś¼Š”¢¼ÓČČ¼°ĘųĢåŹÕ¼Æ×°ÖĆ¾łŅŃĀŌČ„£»±ŲŅŖŹ±æÉŅŌ¼ÓČČ£»a”¢b”¢c”¢d±ķŹ¾ĻąÓ¦ŅĒĘ÷ÖŠ¼ÓČėµÄŹŌ¼Į£©”£
| ĘųĢå | a | b | c | d |
| Cl2 | ÅØŃĪĖį | MnO2 | NaOHČÜŅŗ | ÅØH2SO4 |
| NH3 | ±„ŗĶNH4ClČÜŅŗ | ĻūŹÆ»Ņ | H2O | ¹ĢĢåNaOH |
| NO | Ļ”HNO3 | ĶŠ¼ | H2O | P2O5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģø£½ØŹ”øßČżµŚŅ»“ĪÕļ¶Ļ²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø9·Ö£©ÄāÓĆĻĀĶ¼×°ÖĆÖĘČ”±ķÖŠµÄĖÄÖÖøÉŌļ”¢“æ¾»µÄĘųĢå£ØĶ¼ÖŠĢś¼ÜĢØ”¢Ģś¼Š”¢¼ÓČČ¼°ĘųĢåŹÕ¼Æ×°ÖĆ¾łŅŃĀŌČ„£»±ŲŅŖŹ±æÉŅŌ¼ÓČČ£»a”¢b”¢c”¢d±ķŹ¾ĻąÓ¦ŅĒĘ÷ÖŠ¼ÓČėµÄŹŌ¼Į£©£®
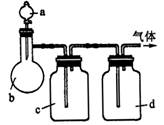
|
ĘųĢå |
a |
b |
c |
d |
|
|
ŅŅ“¼ |
ÅØ |
|
ÅØ |
|
|
ÅØŃĪĖį |
|
|
ÅØ |
|
|
±„ŗĶ |
ĻūŹÆ»Ņ |
|
¹ĢĢå |
|
NO |
Ļ” |
ĶŠ¼ |
|
|
£Ø1£©ÉĻŹö·½·ØÖŠæÉŅŌµĆµ½øÉŌļ”¢“æ¾»µÄĘųĢåŹĒ____________£®
£Ø2£©Öø³ö²»ÄÜÓĆÉĻŹö·½·ØÖĘČ”µÄĘųĢ壬²¢ĖµĆ÷ĄķÓÉ£ØæÉŅŌ²»ĢīĀś£©
¢ŁĘųĢå____________£¬ĄķÓÉŹĒ____________£®
¢ŚĘųĢå____________£¬ĄķÓÉŹĒ____________£®
¢ŪĘųĢå____________£¬ĄķÓÉŹĒ____________£®
¢ÜĘųĢå____________£¬ĄķÓÉŹĒ____________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ¹ć¶«Ź”øßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©ÄāÓĆĻĀĶ¼×°ÖĆÖĘČ”±ķÖŠµÄĖÄÖÖøÉŌļ”¢“æ¾»µÄĘųĢå£ØĶ¼ÖŠĢś¼ÜĢØ”¢Ģś¼Š”¢¼ÓČČ¼°ĘųĢåŹÕ¼Æ×°ÖĆ¾łŅŃĀŌČ„£»±ŲŅŖŹ±æÉŅŌ¼ÓČČ£»a”¢b”¢c”¢d±ķŹ¾ĻąÓ¦ŅĒĘ÷ÖŠ¼ÓČėµÄŹŌ¼Į£©”£
|
ĘųĢå |
a |
b |
c |
d |
|
Cl2 |
ÅØŃĪĖį |
MnO2 |
NaOHČÜŅŗ |
ÅØH2SO4 |
|
NH3 |
±„ŗĶNH4ClČÜŅŗ |
ĻūŹÆ»Ņ |
H2O |
¹ĢĢåNaOH |
|
NO |
Ļ”HNO3 |
ĶŠ¼ |
H2O |
P2O5 |
(1)Š“³öÖĘČ”Cl2µÄ»Æѧ·½³ĢŹ½________________________”£
Š“³öÖĘČ”NOµÄ»Æѧ·½³ĢŹ½________________________”£
(2)ÉĻŹö·½·ØÖŠæÉŅŌµĆµ½øÉŌļ”¢“æ¾»µÄĘųĢåŹĒ ”£
(3)Öø³ö²»ÄÜÓĆÉĻŹö·½·ØÖĘČ”µÄĘųĢ壬²¢ĖµĆ÷ĄķÓÉ£ØæÉŅŌ²»ĢīĀś£©
¢ŁĘųĢå £¬ĄķÓÉŹĒ ”£
¢ŚĘųĢå £¬ĄķÓÉŹĒ ”£
¢ŪĘųĢå £¬ĄķÓÉŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com