
��ȡm gþ���Ͻ���һ��Ũ�ȵ�ϡ������ǡ����ȫ�ܽ�(����Ļ�ԭ����ֻ��NO)����Ӧ��Ļ����Һ�еμ�b mol/L NaOH��Һ�����μӵ�V mLʱ���õ���������ǡ��Ϊ���ֵn g���������йظ�ʵ���˵������ȷ����(����)
�ٳ�����OH��������Ϊ(n��m)g
��ǡ���ܽ����Һ�е�NO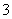 �����ʵ���Ϊ
�����ʵ���Ϊ mol
mol
�۷�Ӧ������ת�Ƶĵ�����Ϊ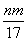 mol
mol
�ܱ�״��������NO�����Ϊ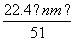 L
L
����Ͻ�Ӧ����������ʵ���Ϊ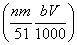 mol
mol
A��5�� B��4��
C��3�� D��2��
�����������漰�ķ�Ӧ��Al��4HNO3(ϡ)===Al(NO3)3��NO����2H2O��3Mg��8HNO3(ϡ)===3Mg(NO3)2��2NO����4H2O��Al(NO3)3��3NaOH===Al(OH)3����3NaNO3��Mg(NO3)2��2NaOH===Mg(OH)2����2NaNO3�����Ͻ�ǡ���ܽ�ʱ����Һ�е�NO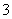 ��Na�������ʵ�����ȣ�n(NO
��Na�������ʵ�����ȣ�n(NO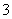 )��n(NaOH)��
)��n(NaOH)�� mol������ȷ�������������ʱ�����ɵ�n g����Ϊ����������������þ�����������غ㶨�ɣ�����þ����Ԫ�ص���������m g�����Գ�����������������Ϊ(n��m)g����Ӧ������ת�Ƶĵ���Ϊn(e��)��n(OH��)��
mol������ȷ�������������ʱ�����ɵ�n g����Ϊ����������������þ�����������غ㶨�ɣ�����þ����Ԫ�ص���������m g�����Գ�����������������Ϊ(n��m)g����Ӧ������ת�Ƶĵ���Ϊn(e��)��n(OH��)��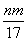 mol���ʢ١�����ȷ�����ݵ��ӵ�ʧ�غ�֪����״����V(NO)��
mol���ʢ١�����ȷ�����ݵ��ӵ�ʧ�غ�֪����״����V(NO)��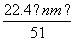 L���ʢ���ȷ���μӷ�Ӧ���������������ã����������õ�����(����������)�����ʵ������������Ƶ����ʵ�������
L���ʢ���ȷ���μӷ�Ӧ���������������ã����������õ�����(����������)�����ʵ������������Ƶ����ʵ������� mol��������������������ʵ�������NO�����ʵ�������
mol��������������������ʵ�������NO�����ʵ�������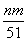 mol�����ԣ���Ͻ�Ӧ����������ʵ���Ϊ
mol�����ԣ���Ͻ�Ӧ����������ʵ���Ϊ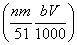 mol���ʢ���ȷ��
mol���ʢ���ȷ��
�𰸡�A



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
��ά����C���л�ԭ�ԣ�������������������
��NO2����ˮʱ����������ԭ��Ӧ����1 mol Cl2�μӷ�Ӧת�Ƶ�����һ��Ϊ2NA���������Ӷ�ֻ�л�ԭ��
A���٢� B���ڢ�
C���ۢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CaCO3(s)===CaO��CO2(g)����H��177.7 kJ
��C(s)��H2O(s)===CO(g)��H2(g)
��H����131.3 kJ/mol
��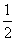 H2SO4(l)��NaOH(l)===
H2SO4(l)��NaOH(l)===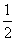 Na2SO4(l)��H2O(l)
Na2SO4(l)��H2O(l)
��H����57.3 kJ/mo l
l
��C(s)��O2(g)===CO2(g)����H����393.5 kJ/mol
��CO(g)��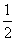 O2(g)===CO2(g)����H����283 kJ/mol
O2(g)===CO2(g)����H����283 kJ/mol
��HNO3(aq)��NaOH(aq)===NaNO3(aq)��H2O(l)
��H����57.3 kJ/mol
��2H2(g)��O2(g)===2H2O(l)
��H����517.6 kJ/mol
(1)�����Ȼ�ѧ����ʽ�У�����ȷ����________������ȷ�����ɷֱ���______________________________________________________________________________________________________________________________��
(2)����������Ϣ��д��Cת��ΪCO���Ȼ�ѧ����ʽ
_________________________________________________________��
(3)������Ӧ�У���ʾȼ���ȵ��Ȼ�ѧ����ʽ��______________����ʾ�к��ȵ��Ȼ�ѧ����ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ������ش��������⣺
(1)��̬Siԭ���У�����ռ�ݵ�����ܲ����Ϊ______�����ܲ���е�ԭ�ӹ����Ϊ______��������Ϊ________��
(2)����Ҫ�Թ����Ρ�________�Ȼ��������ʽ�����ڵؿ��С�
(3)���ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮����________���ϣ��侧���й���8��ԭ�ӣ�����������λ�ù���________��ԭ�ӡ�
(4)���ʹ��ͨ������(SiH4)�ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4Cl��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________________��
(5)̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� | C��C | C��H | C��O | Si��Si | Si��H | Si��O |
| ����/ (kJ��mol��1) | 356 | 413 | 336 | 226 | 318 | 452 |
�ٹ���̼ͬ�壬Ҳ��ϵ���⻯�������������������϶�Զ���������࣬ԭ����___________________________________________________��
��SiH4���ȶ���С��CH4���������������ԭ����
_______________________________________________________��
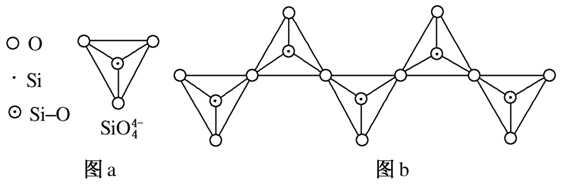
(6)�ڹ������У�SiO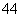 ������(��ͼa)ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ���
������(��ͼa)ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ��� ״�Ĵ���ṹ��ʽ��ͼbΪһ�����������ṹ�Ķ�����������Siԭ�ӵ��ӻ���ʽΪ________��Si��O��ԭ����֮��Ϊ________����ѧʽΪ________��
״�Ĵ���ṹ��ʽ��ͼbΪһ�����������ṹ�Ķ�����������Siԭ�ӵ��ӻ���ʽΪ________��Si��O��ԭ����֮��Ϊ________����ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)�� ���������������õ�ⷨ�ɻ���WC��Co���������̼�ͼ���£�
���������������õ�ⷨ�ɻ���WC��Co���������̼�ͼ���£�
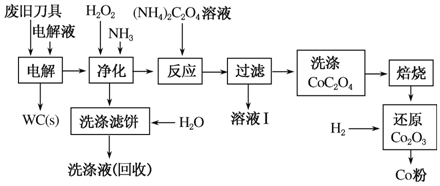
(1)���ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦʽΪ________��
(2)�������������˱�����Ҫ�ɷ���________�����յ�ϴ��Һ����ˮ���Ƶ��Һ��Ŀ���ǻ����������е�________��
(3)��Һ�����Ҫ�ɷ���________��ϴ��CoC2O4����ֶ����ղ�Ʒ���Ȳ�������Ӱ�죬������ʱ����ɻ�����Ⱦ��ԭ����______________��
(4)��Co2O3��ԭ��Co�۵Ļ�ѧ��Ӧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ���ܴ����������(����)
A��pH��1����Һ�У�Fe2����NO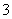 ��SO
��SO ��Na��
��Na��
B��ˮ�������c(H��)��10��12 mol/L����Һ�У�Ca2����K����Cl����HCO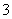
C��c(H��)/c(OH��)��1012��ˮ��Һ�У�NH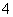 ��Al3����NO
��Al3����NO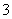 ��Cl��
��Cl��
D��c(Fe3��)��0.1 mol/L����Һ�У�K����ClO����SO ��SCN��
��SCN��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������С����������˷�������ˮ�ȣ��������ʿ����ڼ��ᶾ�Ե���(����)
A����ˮ������������������ B���ƾ�
C����ˮ D��ʳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���л���Ľṹ��ʽΪ��
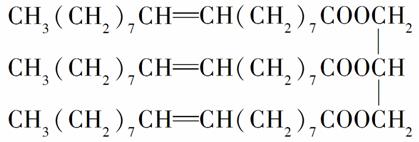
�Իش��������⣺
(1)�û�������________(��ѡ����ĸ����ͬ)��
A��ϩ�� B����֬
C�������� D������
(2)�û�������ܶ�________��
A����ˮ�� B����ˮС
C����ˮ��ͬ
(3)�����¸û������________��
A��Һ̬ B����̬
C����̬
(4)���������У���������ʷ�Ӧ����________��
A��NaOH(aq) B����ˮ
C���Ҵ� D������
E��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��л������ʵ�˵����ȷ����
A�������ʿ������ᡢ����ؽ����η�Ӧ��������B���� ����������ʹ����KMnO4��Һ��ɫ
����������ʹ����KMnO4��Һ��ɫ
C��CH3CH2OH��������ܷ�Ӧ��������������D��ʯ�͵ķ���ɻ����ϩ����ϩ�Ȳ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com