
��5�֣�ijʵ��С����������װ�ã����̶ֹ�װ���ԣ��Ʊ�������(Ca3N2)����̽����ʵ��ʽ��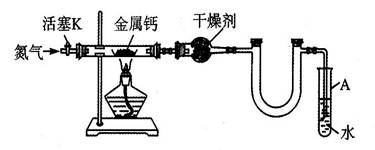
��1����ͼ���Ӻ�ʵ��װ�ã����װ�õ������Եķ�������������������������������
��2����Ӧ������ĩ�˵��ܱ���ʼ�ղ����Թ�A��ˮ�У�Ŀ������������������������
��3���Ʊ������ƵIJ��������Ǣٴ���K��ͨ��N2���ڵ�ȼ�ƾ��ƣ����з�Ӧ��
�۷�Ӧ���������������������ܲ��װ�ã�ȡ�����
��4�����ݼ�¼���£�
| ֱ��������m0/g | ֱ������Ƶ�����m1/g | ֱ��������������m2/g |
| 14.80 | 15.08 | 15.15 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| �մ�������m0/g | ������Ƶ�����m1/g | ��������������m2/g |
| 14.80 | 15.08 | 15.15 |
| 14 |
| 5 |
| 14 |
| 5 |
| n(Ca) |
| n(N) |
| 3 |
| 2 |
| n(Ca) |
| n(N) |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С����������װ��(���̶ֹ�װ����)�Ʊ�������(Ca3N2)����̽����ʵ��ʽ��
(1)��װ���в���U�ܣ�ʵ��Ҳ��˳�����У�ԭ���ǣ�
(2)��Ӧ������ĩ�˵��ܱ���ʼ�ղ����Թ�A��ˮ�У�Ŀ���Ǣٱ��ڹ۲�N2�����٣�
��________
(3)�Ʊ������ƵIJ��������ǣ��ٴ���K��ͨ��N2���ڵ�ȼ�ƾ��ƣ����з�Ӧ���۷�Ӧ������Ϩ��ƾ��ƣ�________
�ܲ��װ�ã�ȡ�����
(4)���ݼ�¼���£�
�ٿմ�������m0/g�� �ڴ�����Ƶ�����m1/g���۴�������������m2/g
�ټ���õ�ʵ��ʽCaxN2������x��________ ����m0��m1��m2��ʾ��
����ͨ���N2�л�������O2������ֵ��ʵ�ʲ��xֵ ���ƫ��ƫС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡ��Դ�ص�һ��ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
ijʵ��С����������װ��(���̶ֹ�װ����)�Ʊ�������(Ca3N2)����̽����ʵ��ʽ��
(1)��װ���в���U�ܣ�ʵ��Ҳ��˳�����У�ԭ���ǣ�
(2)��Ӧ������ĩ�˵��ܱ���ʼ�ղ����Թ�A��ˮ�У�Ŀ���Ǣٱ��ڹ۲�N2�����٣�
��________
(3)�Ʊ������ƵIJ��������ǣ��ٴ���K��ͨ��N2���ڵ�ȼ�ƾ��ƣ����з�Ӧ���۷�Ӧ������Ϩ��ƾ��ƣ�________
�ܲ��װ�ã�ȡ�����
(4)���ݼ�¼���£�
�ٿմ�������m0/g�� �ڴ�����Ƶ�����m1/g���۴�������������m2/g
�ټ���õ�ʵ��ʽCaxN2������x��________ ����m0��m1��m2��ʾ��
����ͨ���N2�л�������O2������ֵ��ʵ�ʲ��xֵ ���ƫ��ƫС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������ʡ�߶���ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ʵ����
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��

�����ķ�Ӧ���£�

��Ӧ��Ͳ������������б����£�
|
|
�е�/�� |
�ܶ�/g��cm-3 |
ˮ���ܽ��� |
|
������ |
117.2 |
0.8109 |
�� |
|
����ȩ |
75.7 |
0.8017 |
�� |
ʵ�鲽�����£�
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����ϵ���֡�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g���ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵������ ��
��2�������ʯ�������� �������Ⱥ���δ�����ʯ��Ӧ��ȡ����ȷ������ ��
��3������װ��ͼ�У�B������������ ��D������������ ��
��4��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ�� �㣨��ϡ����¡�����
��5����Ӧ�¶�Ӧ������90��95�棬��ԭ���� ��
��6����ʵ���У�����ȩ�IJ���Ϊ ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com