
����Ŀ�����������������Ҫ��������������������й㷺Ӧ�ã��Ǵ�����Ҫ��Ⱦ��֮һ������һ���Ļ�ԭ�ԣ�̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��
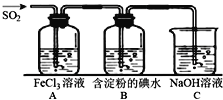
��1��д����ͭ��Ũ������ȡSO2�Ļ�ѧ����ʽ_____________________��
��2��װ��A�е�������__________����Ҫ��A�е�FeCl3��Һ����ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ����ڹ��˲��������õ��IJ���������_______(����)��
A�ƾ��� B��ƿ C©�� D�ձ� E������ F����
��3��������������С��ͬѧ��ΪSO2��FeCl3����������ԭ��Ӧ��
��д��SO2��FeCl3��Һ��Ӧ�����ӷ���ʽ___________________��
�������ʵ�鷽��������Fe2+����__________________________��
��4��B����ɫ��Һ��ɫ������I-�Ļ�ԭ�Ա�SO2__________(����ǿ����������)��
��5����C��������Һֻ��һ��������pH��7������Һ�и�����Ũ�ȴ�С˳��Ϊ_________��
��6����ҵ��ͨ�����ջ��������SO2����һ���õ����ᣬ��֪����1g FeS2����7.1kJ������д������FeS2���Ȼ�ѧ��Ӧ����ʽ____________________��
���𰸡�Cu+2H2SO4 ![]() CuSO4+SO2��+2H2O ��Һ�ɻ�ɫ��Ϊdz��ɫ A��B��F 2Fe3++SO2+2H2O=SO42-+2Fe2++4H+ ȡ����A�з�Ӧ�����Һ���Թ��У�����2��3��K3[Fe(CN)6]��Һ������ɫ�������� �� c(Na+)>c(SO32��)>c(OH��)>c(HSO3��)>c(H+) 4FeS2(s)��11O2(g) = 2Fe2O3(s)��8SO2(g) ��H����3408 kJ/mol
CuSO4+SO2��+2H2O ��Һ�ɻ�ɫ��Ϊdz��ɫ A��B��F 2Fe3++SO2+2H2O=SO42-+2Fe2++4H+ ȡ����A�з�Ӧ�����Һ���Թ��У�����2��3��K3[Fe(CN)6]��Һ������ɫ�������� �� c(Na+)>c(SO32��)>c(OH��)>c(HSO3��)>c(H+) 4FeS2(s)��11O2(g) = 2Fe2O3(s)��8SO2(g) ��H����3408 kJ/mol
��������
��1��Cu��Ũ�����ڼ��������·�Ӧ��������ͭ������������ˮ��
��2�����������Ӿ���ǿ�������ԣ�����Һ�ܹ�������������������������ӣ���������ԭΪ�������ӣ������õ��������У��ձ�����������©����
��3�������������Ӿ���ǿ�������ԣ�����Һ�ܹ�������������������������ӣ���������ԭΪ�������ӣ�
����K3[Fe��CN��6]��Һ���飬����ɫ�������ɣ�
��4��B����ɫ��Һ��ɫ����������������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��I2+SO2+2H2O=4H++2I-+SO42-����ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ�
��5����C��������Һֻ��һ��������pH��7��������ΪNa2SO3��SO32-ˮ����Һ�ʼ��ԣ�����������Դ��SO32-��ˮ�⡢ˮ�ĵ��룻
��6��������Ӧ��4FeS2+11O2=2Fe2O3+8SO2������4molFeS2ȼ�շų���������ע�����ʵľۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
��1��ͭ��Ũ�����ڼ��������·�����Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4 ![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
��2��װ��A��SO2����ԭ����������Ϊ��������ӣ�Fe3+��������������ԭΪFe2+����Ӧ���ӷ���ʽΪ��SO2+2Fe3++2H2O�T2Fe2++SO42-+4H+����A�з�Ӧ������Ϊ��Һ��ɫ�ɻ�ɫ��Ϊdz��ɫ�������õ�©�����ձ�����������û���õ�������ʯ�������������ʴ�ΪABF��
��3����SO2��FeCl3��Һ��Ӧ�����ӷ���ʽ2Fe3++SO2+2H2O=SO42-+2Fe2++4H+��
�ڼ�����Fe2+���ɵIJ�������Ϊȡ����A�з�Ӧ�����Һ���Թ��У�����2��3��K3[Fe(CN)6]��Һ������ɫ�������ɣ�
��4��B����ɫ��Һ��ɫ��˵��SO2��I2��ԭΪI-����֪I-�Ļ�ԭ�Ա�SO2����
��5��NaOH��Һ����SO2��������Һֻ��һ��������pH��7������Һ�е�����ΪNa2SO3��������Ũ�ȴ�С˳��Ϊc(Na+)>c(SO32��)>c(OH��)>c(HSO3��)>c(H+)��
��6��������(��Ҫ�ɷ�ΪFeS2)��ȼ�ղ���ΪSO2��Fe2O3��1g FeS2��ȫȼ�շų�7.1kJ������480gFeS2��ȫȼ�շų�3408kJ��������Ӧ���Ȼ�ѧ����ʽΪ��4FeS2(s)+11O2(g)=2Fe2O3(s)+8SO2(g)��H=-3408kJ/mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
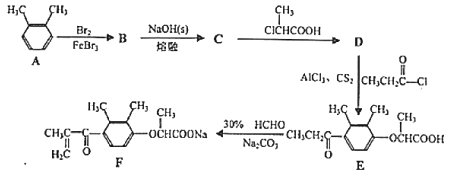
����Ŀ��������F��һ��ҩ��ϳɵ��м��壬F��һ�ֺϳ�·�����£�
��֪��
�ش��������⣺
��1��![]() ������Ϊ____��
������Ϊ____��
��2��D�к��������ŵ�����Ϊ____��
��3��B��C�ķ�Ӧ����ʽΪ____��
��4��D��E�ķ�Ӧ����Ϊ____��
��5��C��ͬ���칹���ж��֣����б�����������ONa��2����CH3��ͬ���칹�廹��____�֣�д���˴Ź�������Ϊ3��壬�����֮��Ϊ6:2:1��ͬ���칹��Ľṹ��ʽ____��
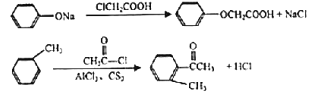
��6�����������ƣ� ����һ�ָ�Ч����ҩ��ο����Ϻϳ�·���е������Ϣ�������
����һ�ָ�Ч����ҩ��ο����Ϻϳ�·���е������Ϣ�������![]() Ϊԭ�ϣ�����ԭ����ѡ���ϳ����������Ƶĺϳ�·�ߡ�________________
Ϊԭ�ϣ�����ԭ����ѡ���ϳ����������Ƶĺϳ�·�ߡ�________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڹ���70��������ϣ����зɹ��ļ��������������Ѫ���ڣ��������й��������Ƶ����Ƚ��ļ�20���γ����ٷɻ����������ٷɻ���ƽ�������ʱ��β���е�NO���ƻ�������,������ͨ���ⶨ�ֲ��ij���Ũ�ȱ仯��ʹ�ɻ�����������������˿�ѧ�������о����ô�������β���е�NO��COת���CO2��N2����ѧ����ʽ���£�2NO(g)+2CO(g) ![]() 2CO2(g)+N2(g) ��H<0����ش��������⣺
2CO2(g)+N2(g) ��H<0����ش��������⣺
(1)�÷�Ӧ��ƽ�ⳣ������ʽΪ___________________________��
(2)�����ڱ���������������Ӧ���ﵽƽ����ȡ����ѡ��Ĵ�ʩ���ܼӿ췴Ӧ�����������NOת���ʵ���_____________��
A.ѡ�ø���Ч�Ĵ���ͬʱ����CO���� B.���߷�Ӧ��ϵ���¶�
C.��ʱ�����ʯ�� D.��С���������
(3)�о���������ʹ�õ���������ʱ����������ȱ���������ѧ��Ӧ���ʡ�Ϊ�˷ֱ���֤�¶ȡ������ȱ�����Ի�ѧ��Ӧ���ʵ�Ӱ����ɣ�ijͬѧ�������ʵ�����NO��CO�����ݻ�Ϊ2L�ĺ����ܱ������У����������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С�
ʵ���� | T/�� | NO��ʼŨ��/mol��L-1 | NOƽ��Ũ��/mol��L-1 | �����ıȱ����/m2��g-1 |
�� | 280 | 0.3 | 0.1 | 82 |
�� | T1 | c1 | c2 | 124 |
�� | 350 | c3 | c4 | 124 |
�������������ʵ�����������У�T1=____________��c3=_____________��
��ƽ��ʱ�������¶�T1�����䣬���������г���CO��CO2��0.2mol����ƽ�⽫_________�ƶ�(��������ҡ�����)

���ڸ���������ͼ�У��������ϱ��е�I��������ʵ�������½���ƽ������У����������NOŨ����ʱ��仯����������ͼ����˵��B���߶�Ӧ��ʵ����__________(��������)��

(4)�ں��º��ݵ��ܱ�������ͨ��n(NO)��n(CO)=1��3�Ļ�����壬����������Ӧ������ͼ����ȷ����˵����Ӧ�ڽ��е�t1ʱ��һ���ﵽƽ��״̬����__________(ѡ����ĸ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����������β���ŷų���NOx��SO2�ȣ����γ���������Ҫ���ʣ����ۺ������ǵ�ǰ��Ҫ���о����⡣
��1����֪����COȼ���ȵ���H1=��283.0kJ��mol-l����N2(g)+O2(g) ![]() 2NO(g) ��H2=+180.5kJ��mol-1������β���е�NO(g)��CO(g)��һ���¶Ⱥʹ��������¿ɷ������·�Ӧ��2NO(g)+2CO(g)
2NO(g) ��H2=+180.5kJ��mol-1������β���е�NO(g)��CO(g)��һ���¶Ⱥʹ��������¿ɷ������·�Ӧ��2NO(g)+2CO(g) ![]() N2(g)+2CO2(g)�� ��H=___��
N2(g)+2CO2(g)�� ��H=___��
��2����0��20mol NO��0��10molCO����һ���ݻ��㶨Ϊ1L���ܱ������з���������Ӧ����Ӧ�����в������ʵ�Ũ�ȱ仯����ͼ��ʾ��
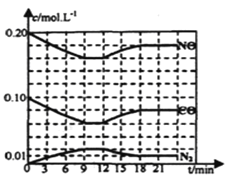
���÷�Ӧ��һ�δﵽƽ��ʱ��ƽ�ⳣ��Ϊ________��
����12minʱ�ı��������________��
���ڵ�24minʱ���������¶Ȳ��䣬���������г���CO��N2��0��060mol��ƽ�⽫________�ƶ�(����������������������������)��
(3)SNCR-SCR����������һ�����͵ij�ȥ�����е������������������һ����ð��������ء�
��SNCR���������У��ڴ�����������NH3����ԭ����ԭNO������Ҫ��ӦΪ��4NH3(g)+4NO(g)+O2(g)=4N2(g)+6H2O(g)����H<0����ϵ�¶�ֱ��Ӱ��SNCR����������Ч�ʣ���ͼ��ʾ������ϵ�¶�ԼΪ925��ʱ��SNCR����Ч����ߣ�����ܵ�ԭ����________��

��SCR������������������[CO(NH2)2]����ԭ����ԭNO2�Ļ�ѧ����ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С��Ϊ�ⶨ������CO2�ĺ���������������ʵ�飺
������0.1000mol��L-1��0.01000mol��L-1�ı����ᡣ
����0.1000mol��L-1�ı�����ζ�δ֪Ũ�ȵ�Ba(OH)2��Һ10.00mL�������ȥ����19.60mL��
���òⶨ��Ba(OH)2��Һ���ն��������е�CO2��ȡBa(OH)2��Һ10.00mL������100mL����ƿ���ˮ���̶��ߣ�ȡ��ϡ�ͺ����Һ�����ܱ������ڣ���ͨ��10L��״���µĿ���������ʱ���ɳ�����
�ܹ�������������Һ��
��ȡ��Һ20.00mL����0.01000mol��L-1������ζ�����ȥ����34.80mL����ش��������⣺
��1�����Ʊ�����ʱ������������Щ����?____��
A.������ƽ B.����ƿ C.��ʽ�ζ��� D.��Ͳ E.�ձ� F.��ͷ�ι� G.������
��2���ζ������У�����____������____���۾�____��
��3��Ba(OH)2��Һ�����ʵ���Ũ����____��
��4������������Һ��Ŀ����____��
��5���˿�����Ʒ�к�CO2���������Ϊ____��
��6����ʵ���У�����һ�εζ�ʱʹ�õ���ʽ�ζ���δ����������ע��ڶ��ֱ����ᣬ�����еڶ��εζ���ʹ�ⶨ���____(�ƫ��ƫС������Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ��������ʵ�����������ȷ���ǣ� ��
A. ��ϩ��һ���������ܷ����ӳɷ�Ӧ���Ӿ۷�Ӧ�������Ը��������Һ����
B. ���Ϸ���ʽΪC3H8O�Ĵ������ֲ�ͬ�ṹ
C. ��ͼ�ļ���ʽ��ʾ��������Ϊ��3-��-4-�һ�-7-������![]()
D. ����ű���������أ���ͼ��������ˮ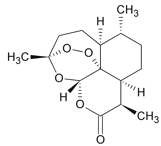
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��
��I2(g)��H2(g)![]() 2HI(g)����H1����9.48 kJ/mol
2HI(g)����H1����9.48 kJ/mol
��I2(s)��H2(g)![]() 2HI(g)����H2����26.48 kJ/mol
2HI(g)����H2����26.48 kJ/mol
�����ж���ȷ����(����)
A.254 g I2(g)��ͨ��2 g H2(g)����Ӧ����9.48 kJ
B.I2(g)=I2(s)����H����17.00 kJ/mol
C.��̬����ȶ��Ա���̬����ȶ��Ը�
D.1 mol��̬���1 mol��̬��ֱ���������ȫ��Ӧ��ǰ�߶��ѵ�I��I������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ��������Na2O���ʵ�Na2O2����������ͼ��ʵ��װ�òⶨNa2O2�����Ĵ��ȡ�(�ɹ�ѡ�õķ�Ӧ��ֻ��CaCO3���塢6 mol��L��1���ᡢ6 mol��L��1���������ˮ)

�ش��������⣺
��1������ͼ��ʾ��װ���������������ڼ�ҩƷ֮ǰӦ�����IJ�����______________
��2��װ��A��Һ���Լ�ѡ��_____________________________��
��3��װ��B��������___________________________________��װ��C��������____________________________________��װ��E�м�ʯ�ҵ�������____________________________________________��
��4��װ��D�з�����Ӧ�Ļ�ѧ����ʽ��______________________________________
��5������ʼʱ�����Ʒ������Ϊ2.0 g����Ӧ���������������Ϊ224 mL(��״��)����Na2O2�����Ĵ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���γɻ�������������Ԫ�أ��䵥�ʼ��γɵĻ����������������������Ҫ��Դ���ʡ�
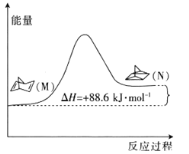
(1)�л���M����̫������տ�ת��������N���������仯��ͼ��ʾ����M��N��ȣ����ȶ�����________(����M������N��)��
(2)��֪��
C(s)��H2O(l)��CO(g)��H2(g) ��H1��a kJ��mol-1
2CO(g)��O2(g)��2CO2(g) ��H2��b kJ��mol-1
2H2(g)��O2(g)��2H2O(l) ��H3��c kJ��mol-1
��C(s)��O2(g)��CO2(g) ��H��______(��a��b��c��ʾ)kJ��mol-1��
(3)���ݼ������ݹ���CH4(g)��4F2(g)��CF4(g)��4HF(g)�ķ�Ӧ����H��_________��
��ѧ�� | C-H | C-F | H-F | F-F |
����(KJmol-1) | 414 | 489 | 565 | 155 |

(4)��һ���ݵ��ܱ������У�����1 mol CO(g)��2 mol H2O(g)��������ӦCO(g)��H2O(g)![]() H2(g)��CO2(g) ��H��CO��ƽ��ת�������¶ȵı仯��ͼ��ʾ��
H2(g)��CO2(g) ��H��CO��ƽ��ת�������¶ȵı仯��ͼ��ʾ��
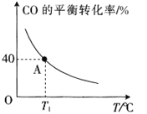
�ٸ÷�Ӧ����H________(����<������>��)0��
�����������ʱ��Ҫ����÷�Ӧ������Ӧ���ʿɲ�ȡ�Ĵ�ʩ��_________(��дһ��)��
��A��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ___________(��ȷ��0.01)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com