
������Ҫ�ĵ��ʣ��Dz����ϴ�Ļ�����Ʒ֮һ���α�����ܵĺϳɰ�������Ϊ���������ǵ¹��˹�����1905�귢���ģ���ϳ�ԭ��Ϊ��
N2(g)��3H2(g)2NH3(g) ��H����92.4 kJ��mol��1
����˻����1918��ŵ������ѧ�����Իش��������⣺
(1)�ϳɰ���ҵ�в�ȡ�����д�ʩ������������ԭ�����͵���________��
A�����ýϸ�ѹǿ(20��50 MPa)
B������500 ��ĸ���
C��������ý������
D�������ɵİ�Һ������ʱ����ϵ�з��������ʣ��N2��H2ѭ�����ϳ���
��������N2��H2
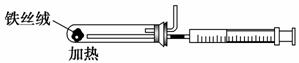
(2)��ͼ��ʵ����ģ�ҵ�ϳɰ��ļ���װ�ã����������а������ɵķ�����
_________________________________________________________________��
(3)��298 Kʱ����10 mol N2��30 mol H2ͨ��ϳ����У��ų�������С��924
kJ��ԭ����_________________________________________________________
_________________________________________________________________��
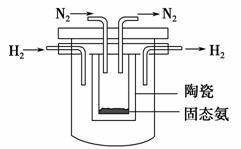
(4)1998��ϣ������˹��´�ѧ��Marmellos��Stoukides���ø����ӵ�����
��SCY�մ�(�ܴ���H��)��ʵ���˸��¡���ѹ�¸�ת���ʵĵ绯ѧ�ϳɰ�����
ʵ��װ������ͼ�����������ĵ缫��ӦʽΪ____________________________��
������(1)��������ԭ��ֻ����ƽ���ƶ����⡣(2)���ݰ������ʽ��м��顣(3)
�ϳɰ��ķ�Ӧ�ǿ��淴Ӧ��(4)���ݺϳɰ���Ӧ�е���������������������
Ӧ�ص㼰����ʳɷֽ��з�����
�𰸡�(1)AD
(2)��ʪ��ĺ�ɫʯ����ֽ���ڹܿڴ�������ֽ������˵���а�������
(3)�÷�Ӧ�ǿ��淴Ӧ��10 mol N2��30 mol H2��������ȫ��Ӧ�����Էų���
������10��92.4 kJ��924 kJ
(4)N2��6H����6e��===2NH3



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������Է�������Ϊ56��
(1)�����ķ���ʽΪ________��
(2)����ʹ����KMnO4��Һ��ɫ������ܵĽṹ��ʽΪ
__________________________________________________________________
_________________________________________________________________��
(3)����(2)�еĻ����������H2��Ӧ�����ò��ﹲ��________�֡�
(4)����(2)�еĻ������������HBr��Ӧ�����ò��ﹲ��________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�������Һ�е���Ũ�ȹ�ϵ��ȷ���� (����)��
A��������ˮ�м������NaOH��c(Na��)��c(Cl��)��c(ClO��)��c(OH��)
B��pH��8.3��NaHCO3��Һ��c(Na��)��c(HCO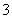 )��c(CO
)��c(CO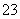 )��c(H2CO3)
)��c(H2CO3)
C��pH��11�İ�ˮ��pH��3������������ϣ�c(Cl��)��c(NH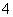 )��c(OH��)��c(H��)
)��c(OH��)��c(H��)
D��0.2 mol��L��1 CH3COOH��Һ��0.1 mol��L��1 NaOH��Һ�������ϣ�2c(H��)��2c(OH��)��c(CH3COO��)��c(CH3COOH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������25 ��ʱ�������ܵ���ʵ��ܽ�ȣ�
| ���ܵ���� | Mg(OH)2 | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
| �ܽ��/g | 9��10��4 | 1.7��10��6 | 1.5��10��4 | 3.0��10��9 |
������������ᴿ�У����������ܵ���ʵ��ܽ�ƽ��ԭ����ȥijЩ���ӡ����磺
��Ϊ�˳�ȥ�Ȼ���е�����Fe3�����Ƚ����������ˮ���ټ���һ�������Լ���Ӧ�����ˣ��ᾧ���ɣ�
��Ϊ�˳�ȥ�Ȼ�þ�����е�����Fe3�����Ƚ����������ˮ������������������þ����ַ�Ӧ�����ˣ��ᾧ���ɣ�
��Ϊ�˳�ȥ����ͭ�����е�����Fe2�����Ƚ����������ˮ������һ������H2O2����Fe2��������Fe3����������Һ��pH��4�����˽ᾧ���ɡ�
��ش��������⣺
(1)�����������ӷ������ܹ��ﵽ�ܺ�Ч����Fe2����Fe3������ת��Ϊ________(������)����ȥ��
(2)���м�����Լ�Ӧѡ��________Ϊ�ˣ���ԭ����_________________��
(3)���г�ȥFe3�����������ܷ�Ӧ�����ӷ���ʽΪ_______________________
_________________________________________________________________��
(4)���й��ڷ�������ص������У���ȷ����________(����ĸ)��
A��H2O2����ɫ�������������������в��������ʣ���������Ⱦ
B����Fe2������ΪFe3������Ҫԭ����Fe(OH)2������Fe(OH)3�������ѹ���
C��������ҺpH��4��ѡ����Լ���������ͭ���ʽ̼��ͭ
D��Cu2�����Դ���������pH��4����Һ��
E����pH>4����Һ��Fe3��һ�����ܴ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳ�NH3�����H2����ú��H2O��Ӧ�Ƶã�������һ����ӦΪCO(g)��H2O(g)CO2(g)��H2(g)����H<0�������COת���ʿɲ��õķ��������У��ٽ����¶ȣ�������ѹǿ����ʹ�ô�����������CO��Ũ�ȣ�������ˮ������Ũ�ȣ�������ȷ������� (����)��
A���٢ڢ� B���ܢ�
C���٢� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A. ��ˮ�������ƶ�����Ư�����ã���Ư��ԭ������
B. ��Ͷ������趼����Ҫ�İ뵼�����
C. �����ʺ���֬��ˮ�ⶼ���ɸ߷��ӻ���������С���ӻ�����Ĺ���
D. MgO��Al2O3�ڹ�ҵ�������������²��ϣ�Ҳ���ڵ�ⷨұ��þ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Һ̬����ӵ����Li+Ƕ�뻯����Ķ��ε�ء���������﮻�����LiCoO2����������̼�缫�������Ϊﮣ�̼��仯����LixC6��0��x��1���������Ϊ�ܽ������LiPF6��LiAsF6�ȵ��л���Һ�������й�˵���������
A��LiCoO2��дΪ���������ʽΪ��Li2O·Co2O3
B���õ�صĵ��Һʹ���л��ܼ���Ҫ�������л��ܼ�������кܺõĻ�����
C���õ�س��ʱ�ķ�ӦΪ��Li++6C+xe—=LixC6+��1-x��Li+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʵ���ɫ��Һ�����������Լ����ɼ������( )
��KOH Na2SO4 AlCl3 ��NaHCO3 Ba(OH)2 H2SO4
��HCl NaAlO2 NaHSO4 ��Ca(OH)2 Na2CO3 BaCl2
A���٢� B���ڢ� C���٢ۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й���ɫ�仯������ǣ� ��
A����4mL0.1mol/L��K2Cr2O7��Һ�еμ�����1mol/LNaOH��Һ����Һ��ɫ�ӳ�ɫ��ɻ�ɫ
B�����Թ��м��������Ȼ��ܾ��壬�μ�Ũ�����ܽ���ˮϡ������ɫ�����Թ�������ˮ��Ƭ�̣���Һ��ɫ��ɷۺ�ɫ
C����Ѫ��ɫ��Fe��SCN��3��Һ�м�������KI���壬��Һ��ɫ��dz
D����50mL��Ͳ��ȡ30mL����ɫ��NO2���岢��סע��ף���������ѹ������ѹ����Ͳ�е����壨�˹����в������¶ȱ仯��������Ͳ���˹۲죬������ɫ��dz
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com