
(1)ͬ��ͬѹ�£�ͬ�����NH3��H2S�������������___________��ͬ������NH3��H2S������������__________��ͬ������NH3��H2S������������ԭ�Ӹ�������___________��������������ԭ�Ӹ�����ȣ����ǵ����ʵ�������________��
(2)Na2SO4??10H2O��Ħ��������__________��483gNa2SO4??10H2O������Na2SO4??10H2O�����ʵ�����_______������Na�������ʵ�����_________������H2O���ӵ���Ŀ��_______����
��ÿ��1�֣���8�֣� ��1��1:2 ��2:1 ��3:1 �� 2:3��2��322g/mol��1.5mol�� 3 mol ��9.03��1024
���������������1�����ݰ����ӵ����ɿ�֪��ͬ��ͬѹ�£�ͬ�����NH3��H2S�������ʵ�����ȣ������������17:34��1:2��ͬ������NH3��H2S������������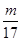 :
: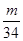 ��2:1��ͬ������NH3��H2S������������ԭ�Ӹ�������
��2:1��ͬ������NH3��H2S������������ԭ�Ӹ�������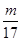 ��3:
��3: 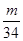 ��2��3:1��������������ԭ�Ӹ�����ȣ�����ݷ���ʽ��֪�����ǵ����ʵ�����2:3��
��2��3:1��������������ԭ�Ӹ�����ȣ�����ݷ���ʽ��֪�����ǵ����ʵ�����2:3��
��2��Na2SO4??10H2O��Ħ�������ǣ�142+180��g/mol��322g/mol ��483gNa2SO4??10H2O������Na2SO4
??10H2O�����ʵ�����483g��322g/mol��1.5mol������Na�������ʵ�����1.5mol��2��3.0mol������H2O���ӵ���Ŀ��1.5mol��10��6.02��1023/mol9.03��1024����
���㣺�������ʵ������йؼ���


 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�̷���FeSO4��7H2O���ڻ�ѧ�ϳ���������ԭ������������ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷���
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ ����>��<��=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO3 20�ˣ�����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL2mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS������������������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����ag�Ȼ�������1.8Lˮ�У�ǡ��ʹ����������ˮ������֮��Ϊ1:100����aֵΪ ��
��2���ڷ�Ӧ2A+B=3C+2D�У���֪3.4gA��3.2gB��ȫ��Ӧ������4.8gC����֪��D��ʽ��Ϊ18����B��ʽ����
��3��25.4g ij���۽����Ȼ���(ACl2)�к���0.4mol Cl������ACl2��Ħ�������� ��A�����ԭ�������� ��ACl2�Ļ�ѧʽ�� ��
��4�� ij�������Na2SO4��Al2��SO4��3��ɣ���֪Na��Al��Ԫ�ص�����֮��Ϊ23: 9����Na2SO4��Al2��SO4��3���ʵ���֮��Ϊ ����1.00mol SO42�C�ĸû���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����Ҫ0.10mol/LNaOH��Һ470mL��������Һ����������ش��������⣺
��1��ʵ���г���������ƽ���ձ�������������Ͳ��ҩ�����Ҫ�����������У� ��
��2�����ݼ����֪������NaOH������Ϊ g��
��3������ʱ��������ƿ����Һ�İ�Һ��������̶������У��Ǻ�ƿ�������һ�������� ��
��4������ʱ,�������ˮ�����̶��ߣ������ȡ�Ĵ�ʩ�ǣ� ��
��5�����в���������Ũ���к�Ӱ�죨��д��ĸ��
ƫ�͵��� ����Ӱ����� ��
| A������������������룻 |
| B����NaOH����ֽ���ϳ����� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У� |
| D������ʱ���ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����Ȼ���MCl2 27g,����0.40molCl-,����Ȼ�������ʵ���Ϊ______,Ħ������Ϊ ������M�����ԭ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ҫȷ���ջ�ѧ���P���ü�����������Ҫ��ش��������⣺
��1��NA��ʾ�����ӵ�������28g��ϩ�ͻ����飨C4H8���Ļ�������к���____NA��̼ԭ�ӣ���������ΪNA����NO2��CO2������庬______ NA����ԭ������1mol37Cl�У�����������������_______ NA����1L 1mol/LFe2(SO4)3��Һ�к�_____NA��SO42�����ӡ�
��2��ͼ��A��B�ֱ���ij���Ľṹʾ��ͼ���ش��������⣺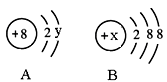
����A��ʾijԪ�ص�ԭ�ӣ���y�� ��
����B��ʾijϡ������Ԫ�ص�ԭ�ӣ����Ԫ�صĵ��ʵĻ�ѧʽΪ ����B�������ӵĽṹʾ��ͼ����x��ȡֵ��Χ��________________��
��3��RxO42����R�Ļ��ϼ�Ϊ___________(�ú�x ��ʽ�ӱ�ʾ)����0.3 mol RxO42����ȫ��Ӧ������RO2ʱ��ת��0.6 mol���ӣ���x��__________��
��4����7.8 gþ���Ͻ���100mL ϡ����ǡ����ȫ��Ӧ������Ӧ�����Һ�������ɣ��õ���ˮ������46.2 g����ԭ��������ʵ���Ũ��Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������NaOH��������250mL 1��25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������ʱ����IJ��������У��ձ����������� �� ��
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������ƽȷ��ȡ�����NaOH����������������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C��������ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3���������Ƶ���ҺŨ��ƫ�͵��� ��
A������NaOHʱ���������������
B��������ƿ��ת����Һʱ(ʵ�鲽��C)������Һ����������ƿ����
C��������ˮʱ���������˿̶���
D������ʱ���ӿ̶���
E������ǰ������ƿ������������ˮ
��4��ijͬѧ���ù���Na2CO3����Na2CO3��Һ�Ĺ�����ͼ��ʾ��������������� 

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ���������Ͻ���100mLijŨ�ȵ������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol��L��1������������Һ����������������Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ��C��0����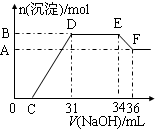
�Իش��������⣺
��1��д����Ӧ������DE�ε����ӷ�Ӧ����ʽ�� ��EF�����ɺ���Ԫ�����ӵ������� ��
��2���Ͻ��У���������Ϊ g����������Ϊ g ��
��3��C��ֵΪ mL��
��4��������Һ�����ʵ���Ũ��Ϊ mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��п��(��Ҫ�ɷ�ΪZnS)�Ǻ�п��Ҫ����֮һ�����¼�����п������ZnO��SO2��ZnO����ұ������п��SO2�����������λ����ᡣ����ش���������(����2λС��)
��1��ȡ1.56 g��п����Ʒ���ڿ����и��¼���(���ʲ���Ӧ)����ַ�Ӧ����ȴ���õ��������������Ϊ1.32 g����Ʒ�к���п������������_________��
��2��ȡ1.95 gп���뵽12.00 mL 18.4 mol/L��Ũ������(�������ɵ���������������Ũ���������һ�Ļ�ԭ����)����ַ�Ӧ��С�ĵؽ���Һϡ�͵�1000 mL��ȡ��15.00 mL���Է�̪Ϊָʾ������0.25 mol/L��NaOH��Һ�ζ�������NaOH��Һ�����Ϊ21.70 mL��ͨ������ȷ��Ũ���ᱻ��ԭ�IJ�����________��
��3������ZnS����ǿ��ɲ����������塣��120 oC��1 atm�£���100 mL����������Ļ�������ȼ���ָ���ԭ��״̬�����ʣ������Ϊ70 mL����ԭ����������������������������������������������ȷֽ⣩
��4���������4.48 L SO2��������ͨ��200 mLһ��Ũ��NaOH��Һ�У�SO2����ȫ�������գ�����Ӧ�����Һ�ڿ�����С�����ɣ���������ʽ�εķֽ⣩���õ��������������ʵ���ˮ����26.8g��ͨ������ȷ�����þ���ijɷ������ʵ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com