
���� ��1����Ӧ2KNO3+3C+S=K2S+N2��+3CO2���У�N��SԪ�صĻ��ϼ۽��ͣ�CԪ�صĻ��ϼ����ߣ���Ԫ�ػ��ϼ۽��͵�����Ϊ������������������ԭ������Ԫ�ػ��ϼ����ߵ�����Ϊ��ԭ������ԭ������������Ӧ�IJ���Ϊ�������
��2������4.48L����״���£���������ʱ�����ݻ��ϼ۱仯����ת�Ƶ��ӵ����ʵ�����
��� �⣺��1���ڷ�Ӧ2KNO3+3C+S=K2S+N2��+3CO2���У�NԪ�صĻ��ϼ���+5����Ϊ0��SԪ�صĻ��ϼ���0����Ϊ-2�ۣ���KNO3��S��������������ԭ��Ԫ��ΪN��S�����ɻ�ԭ����ΪK2S��N2��
CԪ�صĻ��ϼ���0���ߵ�+4����C����ԭ��������������Ӧ��CO2Ϊ�������
�ʴ�Ϊ��N��S��C��KNO3��S��C��CO2��K2S��N2��
��2������4.48L����״���£���0.2mol��������ʱ����0.6molC������ת�Ƶ��ӵ����ʵ���Ϊ0.6����4-0��=2.4mol���ʴ�Ϊ��2.4mol��
���� ���⿼��������ԭ��Ӧ����ȷԪ�صĻ��ϼ۵ı仯��������ԭ��Ӧ�еĻ��������ǽ����Ĺؼ����ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3COOH | B�� | HCl | C�� | CaO | D�� | CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ������Na2CO3��Һ��ϣ�Ca2++CO32-�TCaCO3�� | |
| B�� | NH4HCO3��Һ������NaOH��Һ��ϼ��ȣ�NH4++HCO3-+2OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+CO32-+2H2O | |
| C�� | ����ͨ�������ҺCH3COOH+NH3�TCH3COONH4 | |
| D�� | ����������Һ��ϡ���ᷴӦ��Ba2++SO42-+H++OH-�TBaSO4��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
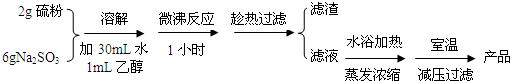
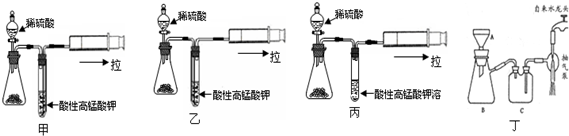
| ��� | ��Ӧ�¶ȣ��棩 | Na2S2O3 | H2O | H2SO4 | ||
| V/mL | c/mol•L-1 | V/mL | V/mL | c/mol•L-1 | ||
| �� | 10 | 10 | 0.1 | 0 | 10 | 0.1 |
| �� | 10 | 5 | 0.1 | 5 | 10 | 0.1 |
| �� | 30 | 10 | 0.1 | 0 | 10 | 0.1 |
| �� | 30 | 5 | 0.1 | 5 | 10 | 0.1 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com