
ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00mL����ƿ�У�����10.00mL KI��Һ��������������ָʾ��2��3�Ρ�
��ȡһֻ��ʽ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01�� mol��L��1 Na2S2O3��Һ������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3��2NaI+Na2S4O6��
�Իش��������⣺
��1������ټ����ָʾ���� ��
��2���ζ�ʱ���۾�Ӧע��____________________��
�жϵ���ζ��յ�������� ,
����ȥNa2S2O3��Һ20.00mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ__________________��
��3��Cl2��ʵ��Ũ�ȱ�����Ũ��Ӧ__________________����ƫ����ƫС������ȡ������������ԭ���� ��������Ϊû�������ʲ���
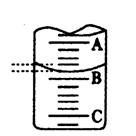
��4����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���� �̶�Ϊ25���ζ�����Һ�����ӦΪ mL�����ʱҺ�������
��Ϊa ml ,�ζ�����Һ������ V ��=���������� �� 50��a ��ml
��5���ζ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNa2S2O3��Һ�����,�ᵼ�²ⶨ��� ����ƫ����ƫС����Ӱ�족����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣�ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00 mL����ƿ�У�����10.00 mL KI��Һ(����)������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01 mol��L-1Na2S2O3��Һ���Լ��ԣ�������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3====2NaI+Na2S4O6��
������������⣺
(1)����ټ����ָʾ����____________________��
(2)�����Ӧʹ��__________ʽ�ζ��ܡ�
(3)����۵�����Һ��___________ɫ��Ϊ__________ɫ�Ҳ��ٱ仯�����յ㣬����ȥNa2S2O3��Һ20.00 mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ____________________��
(4)Cl2��ʵ��Ũ�ȱ�����Ũ��Ӧ_____________(��ƫ��ƫС������ȡ�)���������ԭ����_______________________________��(����Ϊû�������ʲ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00mL����ƿ�У�����10.00mL KI��Һ��������������ָʾ��2��3�Ρ�
��ȡһֻ��ʽ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01�� mol��L��1 Na2S2O3��Һ������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3��2NaI+Na2S4O6��
�Իش��������⣺
��1������ټ����ָʾ���� ��
��2���ζ�ʱ���۾�Ӧע��____________________��
�жϵ���ζ��յ�������� ,
����ȥNa2S2O3��Һ20.00mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ__________________��
��3��Cl2��ʵ��Ũ�ȱ�����Ũ��Ӧ__________________����ƫ����ƫС������ȡ������������ԭ���� ��������Ϊû�������ʲ���
��4����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���� �̶�Ϊ25���ζ�����Һ�����ӦΪ mL�����ʱҺ�������
��Ϊa ml ,�ζ�����Һ������ V ��=���������� �� 50��a ��ml
��5���ζ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNa2S2O3��Һ�����,�ᵼ�²ⶨ��� ����ƫ����ƫС����Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ��Ҧ��ѧ�߶���ѧ�ڵ�һ��������⻯ѧ�Ծ� ���ͣ������
ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00mL����ƿ�У�����10.00mL KI��Һ��������������ָʾ��2��3�Ρ�
��ȡһֻ��ʽ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01�� mol��L��1 Na2S2O3��Һ������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3��2NaI+Na2S4O6��
�Իش��������⣺
��1������ټ����ָʾ���� ��
��2���ζ�ʱ���۾�Ӧע��____________________��
�жϵ���ζ��յ�������� ,
����ȥNa2S2O3��Һ20.00mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ__________________��
��3��Cl2��ʵ��Ũ�ȱ�����Ũ��Ӧ__________________����ƫ����ƫС������ȡ������������ԭ���� ��������Ϊû�������ʲ���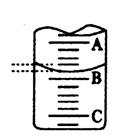
��4����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���� �̶�Ϊ25���ζ�����Һ�����ӦΪ mL�����ʱҺ�������
��Ϊa ml ,�ζ�����Һ������ V ��=���������� �� 50��a ��ml
��5���ζ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNa2S2O3��Һ�����,�ᵼ�²ⶨ��� ����ƫ����ƫС����Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ�߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ�ʵ����
��8�֣�ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00 mL����ƿ�У�����10.00 mL KI��Һ(����)������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01 mol��L-1Na2S2O3��Һ���Լ��ԣ�������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2 + 2Na2S2O3 = 2NaI + Na2S4O6��
������������⣺
(1)����ټ����ָʾ����____________________��
(2)�����Ӧʹ��____________ʽ�ζ��ܡ�
(3)����۵�����Һ��___________ɫ��Ϊ__________ɫ�Ҳ��ٱ仯�����յ㣬����ȥNa2S2O3��Һ40.00 mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ__________________________��
(4)����ʵ�鲽������һ�����ԵIJ�������,��ָ��.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��9�֣�ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00 mL����ƿ�У�����10.00 mL KI��Һ(����)������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.01 mol��L-1Na2S2O3��Һ���Լ��ԣ�������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3====2NaI+Na2S4O6��
������������⣺
(1)����ټ����ָʾ����____________________��
(2)�����Ӧʹ��__________ʽ�ζ��ܡ�
(3)����۵�����Һ��___________ɫ��Ϊ__________ɫ�Ҳ��ٱ仯�����յ㣬����ȥNa2S2O3��Һ20.00 mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ____________________��
(4)Cl2��ʵ��Ũ�ȱ�����Ũ��Ӧ_____________(��ƫ��ƫС������ȡ�)���������ԭ����_______________________________��(����Ϊû�������ʲ���)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com