
����Ԫ��A��B��M��ɵ��������ʷ�����Ӧ����+�� ��+�������м���A��M��ɣ�����B��M��ɣ���ֻ��M��
��1������Ϊ����ɫ���壬�Һͱ���Ϊ�����µ���ɫ��ζ���塣��ĵ���ʽΪ �����ɱ�״����5.6L��ת���Ƶ�����Ϊ �������¶���ҺpH 7�������ӷ���ʽ���� ��
��2������Ϊ��ʹƷ����ɫ����ɫ���壬��Ϊ�����Ϻ�ɫ������������ס�����ԭ�Ӹ����Ⱦ�Ϊ1��2��M����+1�ۣ���ԭ������B����A����
��A�����ڱ���λ��Ϊ
�ڽ���ͨ��FeCl3��Һ�е�����Ϊ
���ӷ�Ӧ����ʽΪ
����ȷ��д�������ɱ��Ļ�ѧ����ʽ
����MCl2����Һ��ͨ�붡���ɹ۲쵽��ɫ��MC1������д���÷�Ӧ�����ӷ���ʽ ��
��֪ʶ�㡿B1 B3 D1 C5 H3
���𰸽����� (1) �������Ƶ���ʽ��(1��) �� 0.5NA ��3.01��1023 (1��)��
> (1��)�� CO32- + H2O  HCO3- + OH- (1��)
HCO3- + OH- (1��)
��д��д�ڶ���ˮ����ɣ�����д�ڶ������0�֣�
��2���ٵڶ�����VIA�� (1��)��
�� ��Һ�ɻ�ɫ��Ϊdz��ɫ(1��)��2Fe3++SO2+2H2O=2Fe2++SO42—+4H+��2�֣�
��2Cu2O + Cu2S = SO2 + 6Cu��2�֣���
��2Cu2+ + SO2 + 2Cl- + 2H2O = 2CuCl�� + SO42- + 4H+ ��2�֣�
��������1�����ݼ�Ϊ����ɫ������������Ԫ��(A��M)��ɵĻ�����,����֪��Na2O2,�����ʽΪ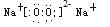 ��������(B��M���)�ͱ�(ֻ����M)��Ϊ�����µ���ɫ��ζ���壬����ǵ��ʣ�����ݷ�Ӧ:��+��=��+�������Ƴ���ΪCO2����ΪO2����ΪNa2CO3����2Na2O2+2CO2= O2��+2Na2CO3֪ÿ2mol Na2O2�μӷ�Ӧ����1mol O2,ת��2mol���ӣ���˱����5��6L O2(��0��25mol)ת�Ƶ�����Ϊ0��5 NA����Na2CO3ˮ��CO32- + H2O
��������(B��M���)�ͱ�(ֻ����M)��Ϊ�����µ���ɫ��ζ���壬����ǵ��ʣ�����ݷ�Ӧ:��+��=��+�������Ƴ���ΪCO2����ΪO2����ΪNa2CO3����2Na2O2+2CO2= O2��+2Na2CO3֪ÿ2mol Na2O2�μӷ�Ӧ����1mol O2,ת��2mol���ӣ���˱����5��6L O2(��0��25mol)ת�Ƶ�����Ϊ0��5 NA����Na2CO3ˮ��CO32- + H2O  HCO3- + OH-ʹ��Һ�Լ��ԣ���pH��7��
HCO3- + OH-ʹ��Һ�Լ��ԣ���pH��7��
��2����������֪��ΪSO2,��ΪCu����A��B��M��S��O��Cu�е�һ�֣��÷�Ӧ�ǻ���ͭ��2Cu2O + Cu2S = SO2 + 6Cu������M����+1��֪M��Cu���ٸ���ԭ������B����A֪AΪO��BΪS��O�����ڱ��ĵڶ�����VIA��ڶ����������Ȼ�����Һ����������ԭ��Ӧ���������Ȼ�����Һ�ɻ�ɫ��Ϊdz��ɫ���Ȼ�ͭ���������Ҳ�ɷ���������ԭ��Ӧ����CuCl������2Cu2+ + SO2 + 2Cl- + 2H2O = 2CuCl�� + SO42- + 4H+��
��˼·�㲦����Ԫ������ֲ�ˮ�⣻���������ƶ������ǽ��ƶ����ͻ�ƿڣ��籾��Ķ�Ϊ��ʹƷ����ɫ����ɫ���壬��Ϊ�����Ϻ�ɫ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ��������ʴ��ʵ�飬һ�ܺ�۲죺
 (1)���Թ���Һ����������������________��ʴ���缫��ӦΪ��
(1)���Թ���Һ����������������________��ʴ���缫��ӦΪ��
����_________________________________________________________��
����___________________________________________________________��
(2)���Թ���Һ���½�����������____________��ʴ���缫��ӦΪ������
________________________________________________________________________��
����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ͼ��ʾװ��(β������������)����ȡһ����̼�������Բⶨijͭ����Ʒ(����CuO��ĩ)�н���ͭ�ĺ�����
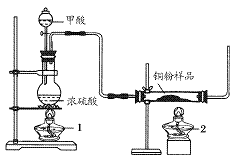
(1)�Ʊ�һ����̼�Ļ�ѧ����ʽ��___________________��
(2)ʵ���У��۲쵽��Ӧ���з�����������_____________��β������Ҫ�ɷ���_________��
(3)��Ӧ��ɺ���ȷ�IJ���˳��Ϊ________(����ĸ)
a���ر�©������ b��Ϩ��ƾ���1 c��Ϩ��ƾ���2
(4)��ʵ���г�ȡͭ����Ʒ10.0 g����ַ�Ӧ��Ӧ����ʣ����������Ϊ9.6 g����ԭ��Ʒ�е���ͭ����������Ϊ________��
(5)��Ũ���ᡢŨ���ᡢ����ˮ��˫��ˮ��ѡ�ú��ʵ��Լ������һ���ⶨ��Ʒ�н���ͭ������������ѷ�����
����Ʒ�������Ҫ������(���������������̵�ϸ��)__________��
��д���йط�Ӧ�Ļ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и��������У�Y������X��Ӧ������Z��Ӧ����
| X | Y | Z | |
| �� | ��ˮ | Al(OH)3 | ����������Һ |
| �� | KOH | SiO2 | ����� |
| �� | �� | SO2 | BaCl2��Һ |
| �� | FeCl3��Һ | Cu | Ũ���� |
A���٢� B���٢� C���ڢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ��������
A��2��4��1 B��3��2��1 C��2��3��1 D��4��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���ǡ�(����)
A.���ؾ���ͬλ��
B.Ԫ�صĽ�����ǿ����Ԫ��ʧȥ�������Ķ��ٳ�����
C.������ͬ����������һ����ͬһ��Ԫ��
D.����������ΪN��R2+,������ΪA,������ӵĺ��������ΪA-N-2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������,����˵������ȷ����(C)
A. ���е���������Ǽ�
B. ��ʽ��������������Ǽ�
C. һ�����ʲ����ܼ��������Ǽ�
D. �������Ǽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȲΪ��Ҫԭ�Ͽ��Ժϳɾ�����ϩ���۱�ϩ����ȶ������Ʊ��������£�
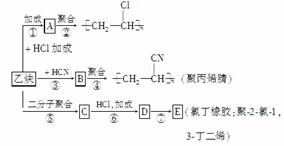
��֪��Ӧ��
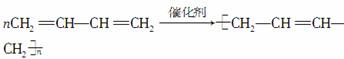
(1)��д��B��C�Ľṹ��ʽ��B______________��C______________________��
(2)д����Ӧ�ۺͷ�Ӧ�ߵĻ�ѧ����ʽ��ָ����Ӧ���͡�
________________________________________________________________________
________________________________________________________________________��
________________________________________________________________________
________________________________________________________________________��
(3)��ij�۱�ϩ���ƽ����Է�������Ϊ26 500����n��________��
(4)��������Ȳ��һ�������¿ɾۺϳɻ�״���ӣ��÷��ӵĽṹ��ʽ�ɱ�ʾΪ
______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.��������(NF3)��һ�����͵��Ӳ��ϣ����ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ���䷴Ӧ�IJ�����:HF��NO��HNO3�������й�˵����ȷ����
A.��Ӧ��NF3����������H2O�ǻ�ԭ��
B.��Ӧ�б������뱻��ԭ��ԭ�����ʵ���֮��Ϊ2:1
C.����Ӧ������0.2 mol HNO3����Ӧ��ת��0.2 mol e-
D. NF3�ڳ�ʪ������й©���������������ɫ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com