
��ͼ��ʾ�����������������ڲ�ͬpH�µ��ܽ�ȣ�S/mol��L��1��������˵������ȷ����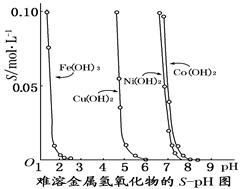
| A��pH��3ʱ��Һ����Ԫ�ص���Ҫ������ʽ��Fe3�� |
| B����Ni(NO3)2��Һ�к���������Co2�����ʣ���ͨ��������ҺpH�ķ�������ȥ |
| C����������Һ�е�Fe3����Cu2�����ɵ�����Һ��pH��4���� |
| D�����ں���Cu2����Ni2������Һ�м����ռNi(OH)2���ȳ��� |
C
�����������������ͼ���֪��pH��3ʱ��Һ����Ԫ�ص���Ҫ������ʽ������������A����ȷ������ͼ���֪������������ȫ����ʱ��pH��������������ȫ����ʱ��pH�����Ե�Co2����ȫ����ʱNi2��Ҳ�Ѿ���ȫ������B����ȷ��pH��4ʱ����������ȫת��Ϊ����������������ͭ���ӻ�������Һ�У�C��ȷ��D����ȷ����Ϊͭ������ȫ����ʱ��pHС������������ȫ����ʱ��pH����ѡC��
���㣺�����ܶȻ��������йؼ��㡢�жϺ�Ӧ��
�����������Ǹ߿��еij���ͼ���ѶȽϴ������ۺ���ǿ��������ͼ��������ѧ���̻���ֱ�����Ϣ�ǻ�ѧ���õı��﷽ʽ��ͼ������ӵ�нϴ����Ϣ�洢�����ܹ���ȫ��ؿ���ѧ���������Ƚϡ����������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013����ͨ�ߵ�ѧУ����ͳһ����(���վ�)��ѧ���� ���ͣ�022
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڣ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�ã�
(1)����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��ã�����Ȼ�ѧ����ʽ���£�2Ca3(PO4)2(s)��10C(s)![]() 6CaO(s)��P4(s)��10CO(g)����H1����3359.26 kJ��mol��1
6CaO(s)��P4(s)��10CO(g)����H1����3359.26 kJ��mol��1
CaO(s)��SiO2(s)![]() CaSiO3(s)����H2����89.61 kJ��mol��1
CaSiO3(s)����H2����89.61 kJ��mol��1
2Ca3(PO4)2(s)��6SiO2(s)��10C(s)![]() 6CaSiO3(s)��P4(s)��10CO(g)����H3��H3��________kJ��mol��1��
6CaSiO3(s)��P4(s)��10CO(g)����H3��H3��________kJ��mol��1��
(2)�����ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P4��60CuSO4��96H2O![]() 20Cu3P��24H3PO4��60H2SO4
20Cu3P��24H3PO4��60H2SO4
60 mol��CuSO4�������������ʵ�����________��
(3)����Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ����ͼ��ʾ��
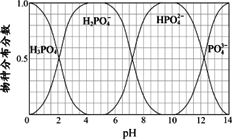
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������________��pH��8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ________��
��Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����________(�����ӷ���ʽ��ʾ)��
(4)�Ļ�������������(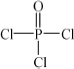 )�뼾���Ĵ�(
)�뼾���Ĵ�( )�����ʵ���֮��2��1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������壮�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
)�����ʵ���֮��2��1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������壮�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
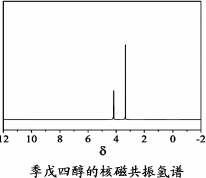
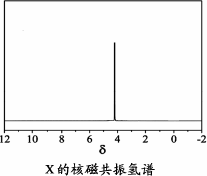
������������________(�ѧʽ)��
��X�Ľṹ��ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���Ի�ѧ�����վ��������� ���ͣ������
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
��1������(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)+10C(s)="==" 6CaO(s)+P4(s)+10CO(g) ��H1 ="+3359.26" kJ��mol��1
CaO(s)+SiO2(s)="==" CaSiO3(s) ��H2 ="-89." 61 kJ��mol��1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s)="==" 6CaSiO3(s)+P4(s)+10CO(g) ��H3
���H3 = kJ��mol��1��
��2�������ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P 4+60CuSO4+96H2O="==" 20Cu3P+24H3PO4+60H2SO4
60molCuSO4�������������ʵ����� ��
��3������Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH �Ĺ�ϵ��ͼ��ʾ��
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������ ��pH=8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ ��
��Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����
(�����ӷ���ʽ��ʾ)��
��4���Ļ�������������( )�뼾���Ĵ�(
)�뼾���Ĵ�( )�����ʵ���֮��2��1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��
)�����ʵ���֮��2��1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��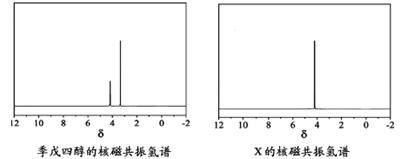
������������ (�ѧʽ)��
��X�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���Ի�ѧ�����վ������棩 ���ͣ������
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
��1������(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)+10C(s)="==" 6CaO(s)+P4(s)+10CO(g) ��H1 ="+3359.26" kJ��mol��1
CaO(s)+SiO2(s)="==" CaSiO3(s) ��H2 ="-89." 61 kJ��mol��1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s)="==" 6CaSiO3(s)+P4(s)+10CO(g) ��H3
���H3 = kJ��mol��1��
��2�������ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P 4+60CuSO4+96H2O="==" 20Cu3P+24H3PO4+60H2SO4
60molCuSO4�������������ʵ����� ��
��3������Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH �Ĺ�ϵ��ͼ��ʾ��
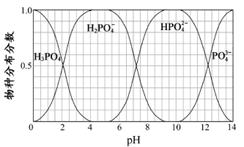
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������ ��pH=8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ ��
��Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����
(�����ӷ���ʽ��ʾ)��
��4���Ļ�������������(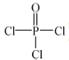 )�뼾���Ĵ�(
)�뼾���Ĵ�( )�����ʵ���֮��2��1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��
)�����ʵ���֮��2��1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��
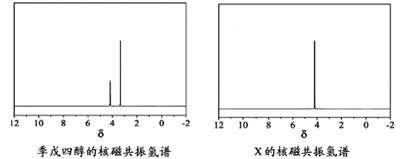
������������ (�ѧʽ)��
��X�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
��1������(P4)����Ca3(PO4)2����̿��SiO2 ��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)+10C(s)=== 6CaO(s)+P4(s)+10CO(g) ��H1 =+3359.26 kJ��mol��1
CaO(s)+SiO2(s)=== CaSiO3(s) ��H2 =-89. 61 kJ��mol��1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s)=== 6CaSiO3(s)+P4(s)+10CO(g) ��H3
���H3 = kJ��mol��1��
��2�������ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P 4+60CuSO4+96H2O=== 20Cu3P+24H3PO4+60H2SO4
 60molCuSO4�������������ʵ����� ��
60molCuSO4�������������ʵ����� ��
��3������Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH �Ĺ�ϵ����ͼ��ʾ��
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������ ��pH=8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ ��
��Na2HPO4 ��Һ�Լ��ԣ�������Һ�м���������CaCl2 ��Һ����Һ�������ԣ���ԭ����
(�����ӷ���ʽ��ʾ)��
��4���Ļ�������������( )�뼾���Ĵ�(
)�뼾���Ĵ�(![]() )�����ʵ���֮��2��1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��
)�����ʵ���֮��2��1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��

������������ (�ѧʽ)��
��X�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com