
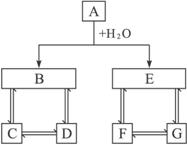
��1��д���������ʵĻ�ѧʽ��A____________��D____________��E____________��G____________��
��2��д�����з�Ӧ�����ӷ���ʽ��
��B��D____________________________________��
��D��C____________________________________��
��C+D��B____________________________________��
��1��Al2S3 NaAlO2 H2S Na2S?
��2����Al��OH��3+OH-====![]() +2H2O ��
+2H2O ��![]() +4H+====Al3++2H2O
+4H+====Al3++2H2O
��Al3++3![]() +6H2O====4Al��OH��3��
+6H2O====4Al��OH��3��
�����������ͻ�ƿ�ΪA��H2O��B��E����ˮ�ܷ�Ӧ�����������ʵ��У�2Na+2H2O====2NaOH+H2����2Na2O2+2H2O====4NaOH+O2����Mg3N2+6H2O====3Mg��OH��2��+2NH3����CaC2+2H2O��Ca��OH��2+CH��CH����Al2S3+6H2O====2Al��OH��3��+3H2S�����ٽ�ϡ�A��E��F��G��ͬһԪ�أ���FΪ���ʡ�������Ϣ��֪��EΪH2S��FΪS��������֪AΪAl2S3��DΪNaAlO2��GΪNa2S��
��Ӧ�����ӷ���ʽΪ����Al��OH��3+OH-====![]() +2H2O��
+2H2O��
��![]() +4H+====Al3++2H2O����Al3++3
+4H+====Al3++2H2O����Al3++3![]() +6H2O====4Al��OH��3����
+6H2O====4Al��OH��3����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
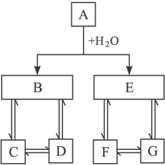
��1��д���������ʵĻ�ѧʽ��
A__________��D__________��E__________��G__________��
��2��д�����з�Ӧ�����ӷ���ʽ��
��B��D______________________________;
��D��C______________________________;
��C+D��B______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��B��C��D��E��F��G��H��Ϊ�л������
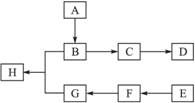
�ش��������⣺
��1���л�������A����Է�������С��60��A�ܷ���������Ӧ��1 mol A�ڴ�������������3 mol H2��Ӧ����B����A�Ľṹ��ʽ��__________����A����B�ķ�Ӧ������__________��
��2��B��Ũ�����м��ȿ�����C��C�ڴ��������¿ɾۺ����ɸ߷��ӻ�����D����C����D�Ļ�ѧ����ʽ��______________________________��
��3���ٷ��㻯����E�ķ���ʽ��C8H8Cl2��E�ı����ϵ�һ��ȡ����ֻ��һ�֣���E�����п��ܵĽṹ��ʽ��____________________��
��E��NaOH��Һ�п�ת��ΪF��F�ø������������Һ��������G��C8H6O4����1 mol G��������NaHCO3��Һ��Ӧ�ɷų�44.8 L CO2(��״��)���ɴ�ȷ��E�Ľṹ��ʽ��________��
��4��G��������B��Ũ������¼��ȷ�Ӧ������H������G��B����H�Ļ�ѧ����ʽ��__________���÷�Ӧ�ķ�Ӧ������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ��㶫ʡ��������ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(22��) I.��ͼ��A��B��C��D��E��F��G��Ϊ�л������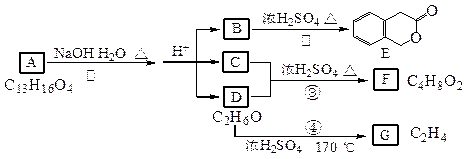
������ͼ�ش����⣺
(1)D�Ļ�ѧ������_____________;B���ṹ��ʽ��_______ _______________;
_______________;
(2)��Ӧ�ܵĻ�ѧ����ʽ��_________________ __________________________;
__________________________;
(3)G����Ҫ�Ĺ�ҵԭ�ϣ�����ѧ����ʽ��ʾG��һ����Ҫ�Ĺ�ҵ��;��________________________________________________________________��
II. һ�ָ߷��ӻ����������Ŀǰ�г������е�ǽ��Ϳ��֮һ����ϳ�·�����£���Ӧ����һ�������½��У���
�ش��������⣺
��1��Ŀǰ��ҵ��������ϩ��Ҫ����ʯ��Ϊԭ��ͨ��________��Ӧ��ʵ�֡�
��2������������ɻ�������ĸ�����Ϊ________________________;
��3��д���ϳ�·���дӻ�������������������Ӧ����ʽ��
___________________________________________________________________;
��4�����й��ڻ�������͢���˵���У��� ȷ���� __
ȷ���� __ __________������ĸ����
__________������ĸ����
A�����������Է���������Ӧ B����������͢�������ʹ������Ȼ�̼��Һ��ɫ
C.��������������������ӳɷ�Ӧ D������������Է���ˮ�ⷴӦ
E��������͢�����������Ʒ�Ӧ�������� F.�����������NaOH ��Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������������ѧ����ģ�⿼�Ի�ѧ�� ���ͣ������
��6�֣�(1)ʵ�����ﲻͬ��ѧ�Լ��ı��淽��������ͬ����ͼ��A��B��C��D�dz�����һЩ����ҩƷ���Լ�ƿ��������г����Լ��������д�ڸ��Լ�ƿ����������ڣ�
(2)����ֽ����NaOH��ҺpH��С�IJ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com