
����Ŀ�����ȵ���NaClO2��һ����Ҫ�ĺ����������������ǹ������ⷨ����NaClO2�Ĺ�������ͼ��
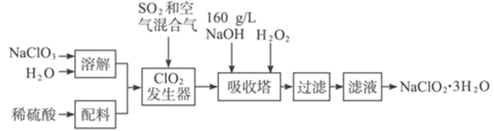
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��ClO2�ķе�Ϊ283 K����ClO2�ֽⱬը������ϡ����������ϡ�ͷ�ֹ��ը�Էֽ�
(1)NaClO2 ������Ϊ_______________
(2)ClO2����������������Ӧ�Ļ�ѧ����ʽΪ_______________���������й�����������ÿ�����_______________��ѡ����ţ���
a����SO2������SO3����ǿ���ԣ� b��ϡ��ClO2�Է�ֹ��ը�� c����NaClO3������ClO2
(3)���չ��������û�ԭ���Ļ�ԭ��Ӧ���У�ԭ����_________����H2O2�⣬������ѡ��Ļ�ԭ����____________������ţ�
A��Na2O2 B��Na2S C��FeCl2 D��KMnO4
(4)����Һ�еõ�NaClO2��3H2O�־����ʵ�����������____________(ѡ�����)��
A������ B������ C������ D������ E����ȴ�ᾧ
(5)�����õ�NaClO2��3H2O�����ñ�ˮϴ�Ӻ�ɣ����ȷ���þ���ϴ�Ӹɾ���
________________________
���𰸡��������� 2NaClO3��SO2��H2SO4��2ClO2��2NaHSO4��2NaClO3��SO2��2ClO2��Na2SO4 b ��ֹ����NaClO2��������ԭ��NaCl A BED ȡ���һ��ϴ��Һ�����������ữ��BaCl2��Һ�����Ƿ��г�������
��������
��ClO2��������NaClO3��H2SO4��SO2����Һ�з�����Ӧ����ClO2��NaHSO4����Ӧ������Һ��ͨ��������ɽ�ClO2�����������У�����NaOH��H2O2��������NaClO2��H2O2����������O2������ȴ�ᾧ���ؽᾧ������õ�NaClO23H2O���Դ˽����⡣
(1)NaClO2��ClԪ�ػ��ϼ�Ϊ+3�ۣ�����Ϊ�������ƣ�
(2)��ClO2��������NaClO3��H2SO4��SO2����Һ�з�����Ӧ����ClO2��NaHSO4����Ӧ�Ļ�ѧ����ʽΪ��2NaClO3��SO2��H2SO4��2ClO2��2NaHSO4����ClO2�ֽⱬը���������й��������������ϡ��ClO2�Է�ֹ��ը���ʺ���ѡ����b��
(3)�������з�����Ӧ�Ļ�ѧ����ʽΪ2ClO2+H2O2+2NaOH�T2NaClO2+2H2O+O2�������չ��������û�ԭ���Ļ�ԭ��Ӧ���У���Ϊ�˷�ֹ����NaClO2��������ԭ��NaCl��Ϊ�����������ʣ���ԭ��Ҳ����Ϊ�������ƣ��ʺ���ѡ����A��
(4)����Һ�еõ�NaClO2��3H2O�־����ʵ���������������Ũ������ȴ�ᾧ�����ˣ��ʺ���ѡ����BED��
(5)�����õ�NaClO2��3H2O�����ñ�ˮϴ�Ӻ�ɣ�������ϴ�Ӹɾ�����ϴ��Һ�в���SO42-����ȷ���þ���ϴ�Ӹɾ��IJ����ǣ�ȡ���һ��ϴ��Һ�����������ữ��BaCl2��Һ�����Ƿ��г������ɣ�������������˵������ϴ�Ӹɾ����������δϴ�Ӹɾ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�л���A����ṹ��ʽ��ͼ����д�����»�ѧ��Ӧ�ķ���ʽ��
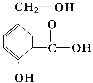
��A��NaOH��Һ��Ӧ��_____________________
��A��NaHCO3��Һ��Ӧ��____________________��
��A��һ�������¸�Na��Ӧ��__________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��һ��ҩ��ϳ��м��壬��ϳ�·�����£�
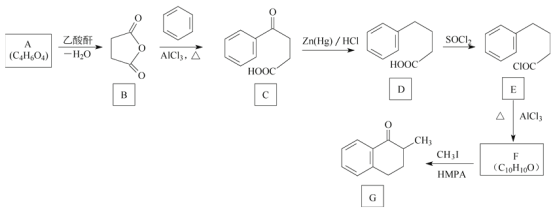
��֪A������ֻ����һ�ֹ�����������NaHCO3��Ӧ����CO2���ش��������⣺
(1)A�Ļ�ѧ������___________��
(2)C�еĹ�����������___________��
(3)D����E�ķ�Ӧ������___________��
(4)F�Ľṹ��ʽ��___________��
(5)A���Ҷ����ڴ��������·�Ӧ���ɿ����オ���;���PES���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
(6)X��G��ͬ���칹�壬��������������X����___________��(�����������칹)�����к˴Ź���������5����������Ϊ6��2��2��1��1����___________(д�ṹ��ʽ)��
�ٺ������ұ�����ֻ������ȡ�������ں�ȩ�����뱽��ֱ���������۳������ⲻ��������״�ṹ��
(7)����ɱ��ͼױ��Ʊ�![]() �ĺϳ�·�ߣ�_________(���Լ���ѡ)��
�ĺϳ�·�ߣ�_________(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦNH4Cl+NaNO2�TNaCl+N2��+2H2O�����Ҳ������壬�����ڶ���ʯ�͵Ŀ��ɡ����б�ʾ��Ӧ��������Ļ�ѧ������ȷ���ǣ� ��
A.������Ϊ18����ԭ�ӣ�![]() Cl
Cl
B.H2O�ĵ���ʽ��![]()
C.Na+�Ľṹʾ��ͼ��![]()
D.N2�Ľṹʽ��N�TN
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ����������ͭ��Һ��ɵ�ԭ����У�����������_______________�������ĵ缫��ӦʽΪ____________________________������������______________��������___________________����������������������ͬ��������������________________�����ܷ�Ӧ�����ӷ���ʽΪ_________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȩ��һ����Ҫ�����ϣ��㷺Ӧ���ڻ�ױƷ��ʳ����ϵ���ҵ��G��һ�ֳ��õ���ȩ�������к���һ����Ԫ����һ����Ԫ���ṹ��G��������ͼ;���ϳɣ�
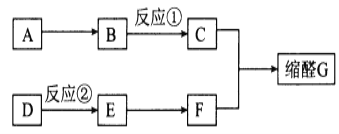
��֪��I��A�ķ���ʽΪC7H8�����ڷ�������D�IJ���������������һ������ʯ�ͻ�����չˮƽ��
����ȩ����Ӧԭ����
RCHO+R��OH![]()
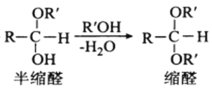
��ͬһ��̼ԭ�����������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺
��1��F������Ϊ__________��
��2����Ӧ�ٵ�����Ϊ___________����Ӧ�ڵķ�Ӧ����Ϊ___________��
��3��д����A����B�Ļ�ѧ����ʽ��________��
��4����ȩG�����й����ŵ�����Ϊ___________��ͬʱ��������������G��ͬ���칹����_____�֣������������칹�������к˴Ź�������Ϊ����壬�ҷ����֮��Ϊ1��1��2��2��2��2�Ľṹ��ʽΪ_____��
�ٱ�����ֻ������ȡ���� �ڼ��ܷ���������Ӧ��������FeCl3��Һ������ɫ��Ӧ
��5��D��һ�ֺ�4��̼ԭ�ӵ���֧��ͬϵ��H������̼ԭ��һ����ͬһƽ���ϡ�H��Ϊԭ�Ͽ�������ȡĿǰ�����ӵڶ�λ��˳������д���úϳ�·�ߣ������Լ���ѡ����_______ ![]() ˳����
˳����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ī�����������ε�һ�ָ��Σ���һ����Ҫ�Ļ�ѧ�Լ�������ˮ��Һ�м���KSCN���Ժ�ɫ��������ͼ��ʾ�ķ�Ӧ��
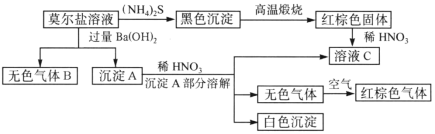
�������Ϲ�ϵ����Ҫ����գ�
(1)���ü�������B__________________��
(2)Ī���εĻ�ѧʽΪ____________________��
(3)���ֳ���A��ϡHNO3��Ӧ�����ӷ���ʽ��_____________________��
(4)�Ŵ�������Ʒ��Ǹ������������̷�(FeSO4��7H2O)����������ȴ���Ƶ�һ����ɫճ����Һ�����̷�������ʣ��Ĺ���Ϊ����ɫ������֪SO3���۵���16.8��C���е���44.8��C���ټӷ����ȷֽ�IJ��

��װ�õ�����˳��Ϊ______________��
��B����������֤�÷�Ӧ��ˮ���ɣ����е��Լ�Ϊ_________��ʵ������з���F����Һ��ɫ��D�г�����ɫճ��Һ�壬�������̷��ķ�Ӧ����ʽΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��þԪ�غ���Ԫ�ص������Ϣ���ɴ˲��ܵõ�����Ϣ�ǣ� ��

A.þ���ڽ���Ԫ��
B.þԭ�Ӻ�����12������
C.þ�����������ӵĵ��Ӳ������
D.�ڻ�ѧ��Ӧ�У�1����ԭ�����õ�2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ�ṹ��˵������ȷ���ǣ� ��
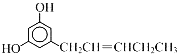
A. �����м���![]() �����Цм�
�����Цм�
B. ̼ԭ����sp��sp2��sp3�����ӻ���ʽ
C. O��H���ļ���ǿ��C-H���ļ���
D. �ǻ�����ԭ�Ӳ�ȡsp3�ӻ���VSEPRģ��Ϊ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com