
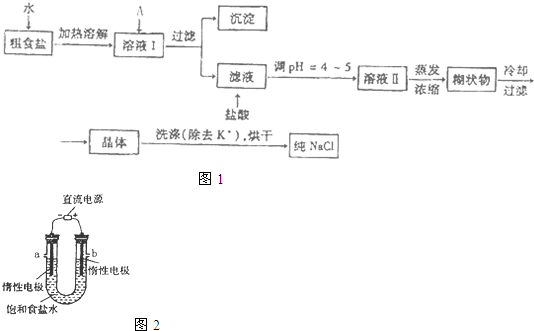
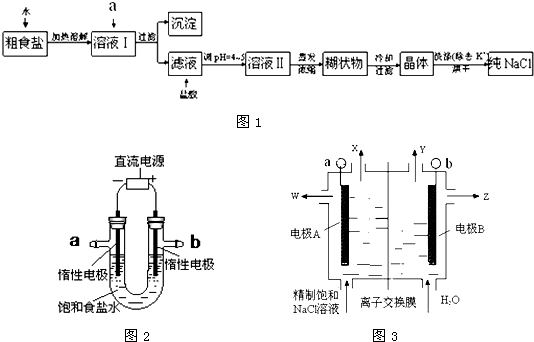
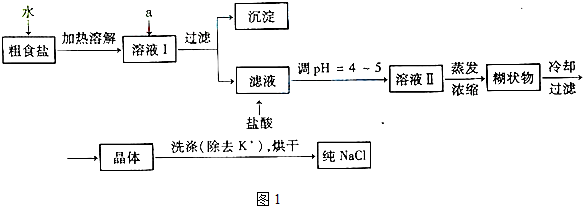
��ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ�� ����K2CO3��Һ ��NaOH��Һ�� KOH��Һ�� BaCl2��Һ ��Ba��NO3��2��Һ
��1������ȥ��Һ�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a�������ĸ����Լ������μ�˳������Ϊ ���ѧʽ����
��2������Ũ����Һ��õ��ĺ�״��Ļ�ѧ�ɷ������ǣ��ѧʽ����
��3�����ᴿ����NaCl����������480 mL 0��4 mol��L-1NaCl��Һʱ�������������ձ���ҩ�ס�����������ƽ����ͷ�ι���� �����������ƣ�����NaCl g��
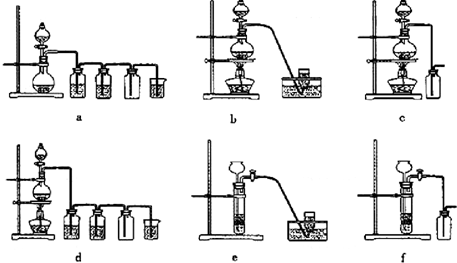
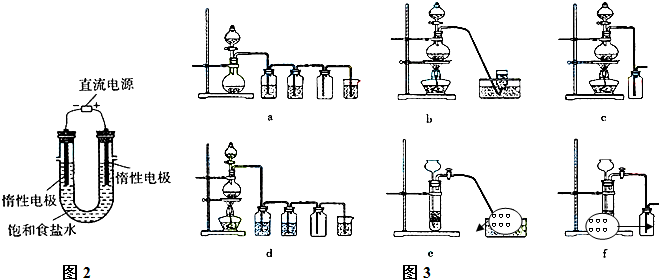
��4����ⱥ��ʳ��ˮ��װ����ͼ��ʾ��
���ռ�����H2Ϊ2 L����ͬ���������ռ�����Cl2��� ���>������=����<����2 L����ԭ���ǣ� ��������������������װ�õ�b���ܷ�ס������һ��ʱ���U���п��Ի��һ������Һ��д����ô�����Һ��һ���ܷ�Ӧ����ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com