
| µĪ¶ØŠņŗÅ | “ż²āŅŗĢå»ż£Øml£© | ĖłĻūŗÄŃĪĖį±ź×¼ŅŗµÄĢå»ż£ØmL£© | |
| µĪ¶ØĒ° | µĪ¶Øŗó | ||
| 1 | 20.00 | 0.50 | 20.55 |
| 2 | 20.00 | 6.00 | 25.95 |
·ÖĪö £Ø1£©µĪ¶Ø¹ż³ĢÖŠ£¬ŃŪ¾¦×¢ŹÓ×Å׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£»øł¾ŻČÜŅŗŃÕÉ«±ä»ÆĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£¬æÉĖµĆ÷“ļµ½µĪ¶ØÖÕµć£»
£Ø2£©ĻČĖć³öĮ½“ĪĻūŗÄŃĪĖįµÄĘ½¾łĢå»ż£¬Č»ŗóĒó³öĒāŃõ»ÆÄʵÄĪļÖŹµÄĮ棬ŌŁ¼ĘĖćÉÕ¼īѳʷµÄ“æ¶Č£»
£Ø3£©¢ŁÓĆÕōĮóĖ®³åĻ“׶ŠĪĘæ¶Ō²ā¶Ø½į¹ūĪŽÓ°Ļģ£»
¢ŚČōµĪ¶ØĒ°ÓĆÕōĮóĖ®³åĻ“ĖįŹ½µĪ¶Ø¹Üŗó¼“×°±ź×¼ŃĪĖį£¬±ź×¼ŅŗµÄÅØ¶Č¼õÉŁ£¬Ģå»żĘ«“󣬲ā¶Ø½į¹ūĘ«“ó£»
¢ŪµĪ¼ÓŃĪĖįĖŁ¶Č¹żæģ£¬Ī“³ä·ÖÕńµ“£¬øÕ擵½ČÜŅŗ±äÉ«£¬Į¢æĢĶ£Ö¹µĪ¶Ø£¬Ōņ»įŹ¹²ā¶Ø½į¹ū£¬ŃĪĖįĢå»żĘ«Š”£¬²ā¶Ø½į¹ūĘ«Š”£®
½ā“š ½ā£ŗ£Ø1£©µĪ¶Ø¹ż³ĢÖŠ£¬ŃŪ¾¦×¢ŹÓ×Å׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£»µĪ¶ØÖÕµćÅŠ¶Ļ£¬×¶ŠĪĘæÄŚČÜÓÉŗģÉ«±äĪŽÉ«£¬°ė·ÖÖÓÄŚĪŽŃÕÉ«±ä»Æ£¬
¹Ź“š°øĪŖ£ŗČÜŅŗÓÉŗģÉ«±äĪŽÉ«£¬°ė·ÖÖÓÄŚĪŽŃÕÉ«±ä»Æ£»
£Ø2£©Į½“ĪĻūŗÄŃĪĖįĢå»ż·Ö±šĪŖ£ŗ20.55ml-0.50ml=20.05ml”¢25.95ml-6.00=19.95£»ĻūŗÄŃĪĖįµÄĘ½¾łĢå»żĪŖ20.00mL£¬n£ØÉÕ¼ī£©=n£ØŃĪĖį£©=0.20mol/L”Į0.02L=0.004mol£¬m£ØÉÕ¼ī£©ØTnMØT0.004mol”Į40g/mol=0.16g£¬ÉÕ¼īµÄ“æ¶Č¦Ų£ØÉÕ¼ī£©=$\frac{\frac{0.16g}{2.0g}}{10}$”Į100%=80%£¬
¹Ź“š°øĪŖ£ŗ80%£»
£Ø3£©¢ŁÓĆÕōĮóĖ®³åĻ“׶ŠĪĘæ¶Ō²ā¶Ø½į¹ūĪŽÓ°Ļģ£»
¢ŚČōµĪ¶ØĒ°ÓĆÕōĮóĖ®³åĻ“ĖįŹ½µĪ¶Ø¹Üŗó¼“×°±ź×¼ŃĪĖį£¬±ź×¼ŅŗµÄÅØ¶Č¼õÉŁ£¬Ģå»żĘ«“󣬲ā¶Ø½į¹ūĘ«“ó£»
¢ŪµĪ¼ÓŃĪĖįĖŁ¶Č¹żæģ£¬Ī“³ä·ÖÕńµ“£¬øÕ擵½ČÜŅŗ±äÉ«£¬Į¢æĢĶ£Ö¹µĪ¶Ø£¬Ōņ»įŹ¹²ā¶Ø½į¹ū£¬ŃĪĖįĢå»żĘ«Š”£¬²ā¶Ø½į¹ūĘ«Š”£»
¹Ź“š°øĪŖ£ŗĪŽÓ°Ļģ£»Ę«øߣ»Ę«µĶ£®
µćĘĄ ±¾Ģāæ¼²éĮĖµĪ¶Ø²Ł×÷£®²Ł×÷Ź±ŅŖ¹ę·¶£¬·ÖĪöĪó²īŹ±ŅŖæ“ŹĒ·ńÓ°Ļģ±ź×¼Ģå»żµÄÓĆĮ棬Čō±ź×¼Ģå»żĘ«“󣬽į¹ūĘ«øߣ»Čō±ź×¼Ģå»żĘ«Š”£¬Ōņ½į¹ūĘ«Š”£»Čō²»Ó°Ļģ±ź×¼Ģå»ż£¬Ōņ½į¹ūĪŽÓ°Ļģ£®


 ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
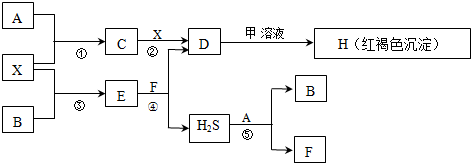
 £®
£®| pH | 1 | 3 | 5 | 7 | 9 | 11 | 13 |
| c£ØS2-£© | 1.4”Į10-19 | 1.4”Į10-15 | 1.4”Į10-11 | 6.7”Į10-7 | 1.9”Į10-5 | 1.3”Į10-3 | 5.7”Į10-2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ū¢Ü | B£® | ¢Ū¢Ü¢Ż¢Ž | C£® | ¢Ś¢Ū¢Ü¢Ž | D£® | Č«²æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaAlO2ŗĶHNO3 | B£® | AlCl3ŗĶKOH | C£® | KHSO4ŗĶBa£ØOH£©2 | D£® | K2CO3ŗĶHCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĶŗĻ½š | B£® | Ļš½ŗ | C£® | ¾ŪŅŅĻ© | D£® | ¶žŃõ»Æ¹č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1mol/LŃĪĖįČÜŅŗÖŠŗ¬H+1mol | |
| B£® | ³£ĪĀ³£Ń¹ĻĀ£¬1mol CH4µÄÖŹĮæĪŖ16g/mol | |
| C£® | 28gC2H4ŗĶC3H6»ģŗĻĘųĢåŌ×ÓŹżÄæĪŖ6NA | |
| D£® | ±ź×¼×“æöĻĀ£¬2.24 L H2OÖŠĖłŗ¬Ō×ÓŹż¾łĪŖ0.3NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
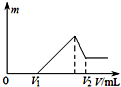
| A£® | 24mL | B£® | 30mL | C£® | 440mL | D£® | 44mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 8ÖÖ | B£® | 6ÖÖ | C£® | 5ÖÖ | D£® | 4ÖÖ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com