
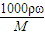 ����Ũ��������ʵ���Ũ�ȣ�
����Ũ��������ʵ���Ũ�ȣ� ����������������ҺŨ�ȵ�Ӱ�죮
����������������ҺŨ�ȵ�Ӱ�죮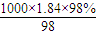 mol/L=18.4mol/L��
mol/L=18.4mol/L��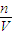 ������Һ����������������
������Һ���������������� 

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ʵ������480mL��0.5 mol/L ��ϡH2SO4��Һ��ijͬѧ��98%��ŨH2SO4����=1.84 g/cm3���������ƣ���ش��������⣺
(1)98%��ŨH2SO4(��=1.84 g/cm3)�����ʵ���Ũ��Ϊ
(2)�����в����еĿո��ڽ���������������д����
����20 mL��Ͳ��ȡ�����Ũ����
�ڽ��ձ��е���Һת�Ƶ� mL������ƿ��
�۽�Ũ���Ỻ��ע��ʢ����������ˮ���ձ��У��ӱ߽���
�ܽ���Һ��ȴ���ָ�����
��������ƿ�м�������ˮ���ھ���̶�1~2 cmʱ������ ������ˮ���̶���
�Ǻ�ƿ�����������µߵ���ҡ��
��ϴ���ձ�2~3�Σ�ϴ��ҺҲע������ƿ�С�����ҡ������ƿ��ʹ��Һ��Ͼ��ȡ�
(3)ʵ���������������ȷ˳��Ϊ (����ţ���
(4)�ں�������д���и����������������ҺŨ�ȵ�Ӱ�죨ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�����õ�Ũ���᳤ʱ��������ܷⲻ�õ�������
����ȡŨ����������Ͳ������ˮ
�۶���ʱ������Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com