����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
��1��������ȼ������ʱ����Ӧ��N
2��g��+O
2��g��=2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���8mol N
2��9molO
2��5min���ƽ��ʱNO ���ʵ���Ϊ6mol���÷�Ӧ������v��NO��Ϊ
���������µ�ƽ�ⳣ����ֵΪ
��2��H
2��CO���Դ���ԭNO�Դﵽ������Ⱦ��Ŀ�ģ�
����֪��N
2��g��+O
2��g��=2NO��g����H=+180.5kJ/mol2H
2��g��+O
2��g��=2H
2O��l����H=-571.6kJ/mol
��H
2��g����NO��g����Ӧ����N
2��g����H
2O��l�����Ȼ�ѧ����ʽΪ
��
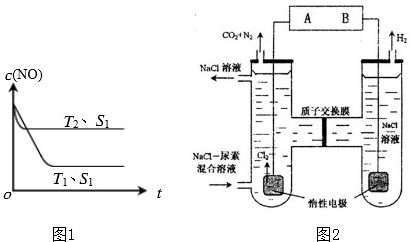
�ڵ�����һ��ʱ�������������ı���������ѧ��Ӧ���ʣ���ͼ1�Ƿ�Ӧ��
2NO��g��+2CO��g��=2CO
2��g��+N
2��g����NO��Ũ�����¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߣ��ݴ��жϸ÷�Ӧ�ġ�H
0 �������������������ȷ���������������ı����������S
2����Ӧ��T
1��S
2�����´ﵽƽ��ʱNO��Ũ��
�����������С�����䡱��
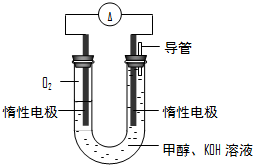
��3���˹�������ü�ӵ绯ѧ������ȥ��л�����е����أ�CO��NH
2��
2������ͨ��������Ӧ���ɾ���ǿ�������õ��м������������л���ԭ����ͼ2��
�ٵ�Դ�ĸ���Ϊ
���A����B������
���������з����ķ�ӦΪ
�۵���������������Һ��pH����ǰ��Ƚ�
�����������ռ�������13.44L����״���������ȥ������Ϊ
g������������ܽ⣩��
��4������ͬ����CO��g����H
2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�ӦCO��g��+H
2O��g��?CO
2��g��+H
2��g�����õ������������ݣ�
| ʵ���� |
�¶ȡ� |
��ʼ��/mol |
ƽ����/mol |
�ﵽƽ������
ʱ��/min |
| CO |
H2O |
H2 |
CO |
| 1 |
650 |
4 |
2 |
1.6 |
2.4 |
6 |
| 2 |
900 |
2 |
1 |
0.4 |
1.6 |
3 |
| 3 |
900 |
a |
b |
c |
d |
t |
��ʵ��2������ƽ�ⳣ��K=
��
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ������ת���ʣ���
��ֵ
�������ֵ��ȡֵ��Χ����
��ʵ��4����900��ʱ���ڴ������м���CO��H
2O��CO
2��H
2��Ϊ1mol�����ʱV
��
V
���������������������=������

 CO2��g��+H2��g��
CO2��g��+H2��g��


 ��2013?����һģ������̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ����
��2013?����һģ������̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ����

 ����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�