
Õż¶”Č©æÉŌŚōŹ»łīÜ“ß»ÆĻĀÓɱūĻ©Ēā¼×õ£»Æ»ńµĆ£ŗCH3CH=CH2+CO+H2 CH3CH2CH2CHO£ØÕż¶”Č©£©ø±·“Ó¦£ŗCH3CH=CH2+CO+H2
CH3CH2CH2CHO£ØÕż¶”Č©£©ø±·“Ó¦£ŗCH3CH=CH2+CO+H2 £ØCH3£©2CHCHO£ØŅģ¶”Č©£©£¬ĪĀ¶Č¶Ō±ūĻ©Ēā¼×õ£»Æ²śĪļÖŠÕż/ŅģČ©±ČµÄÓ°ĻģČēĻĀĶ¼£ŗ
£ØCH3£©2CHCHO£ØŅģ¶”Č©£©£¬ĪĀ¶Č¶Ō±ūĻ©Ēā¼×õ£»Æ²śĪļÖŠÕż/ŅģČ©±ČµÄÓ°ĻģČēĻĀĶ¼£ŗ
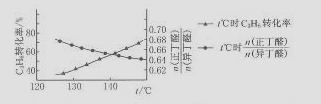
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £Ø £©
A£®±ūĻ©Ēā¼×õ£»Æ·“Ó¦ŹĒ·ÅČČ·“Ó¦
B£®±ūĻ©ÓėCO4H2»ģŗĻ“ß»ÆŹ±£¬æÉÄÜÓŠ±ūĶé¼°Õż¶”“¼µČø±²śĪļÉś³É
C£®ĻąĶ¬ĪļÖŹµÄĮæµÄ±ūĻ©ŗĻ³ÉÕż¶”Č©Ź±£¬ĪĀ¶ČŌ½øߣ¬Ėł»ńµĆµÄÕż¶”Č©µÄ²śĮæŅ»¶ØŌ½“ó
D£®ĪĀ¶Č²»±äŹ±£¬Ö»øıäĢåĻµµÄŃ¹Ē棬¶Ō²śĀŹĆ»ÓŠÓ°Ļģ
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Õż¶”Č©æÉŌŚōŹ»łīÜ“ß»ÆĻĀÓɱūĻ©Ēā¼×õ£»Æ»ńµĆ£ŗCH3CH=CH2+CO+H2![]() CH3CH2CH2CHO£ØÕż¶”Č©£©ø±·“Ó¦£ŗCH3CH=CH2+CO+H2
CH3CH2CH2CHO£ØÕż¶”Č©£©ø±·“Ó¦£ŗCH3CH=CH2+CO+H2![]() £ØCH3£©2CHCHO£ØŅģ¶”Č©£©£¬ĪĀ¶Č¶Ō±ūĻ©Ēā¼×õ£»Æ²śĪļÖŠÕż/ŅģČ©±ČµÄÓ°ĻģČēĻĀĶ¼£ŗ
£ØCH3£©2CHCHO£ØŅģ¶”Č©£©£¬ĪĀ¶Č¶Ō±ūĻ©Ēā¼×õ£»Æ²śĪļÖŠÕż/ŅģČ©±ČµÄÓ°ĻģČēĻĀĶ¼£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £Ø £©
A£®±ūĻ©Ēā¼×õ£»Æ·“Ó¦ŹĒ·ÅČČ·“Ó¦
B£®±ūĻ©ÓėCO4H2»ģŗĻ“ß»ÆŹ±£¬æÉÄÜÓŠ±ūĶé¼°Õż¶”“¼µČø±²śĪļÉś³É
C£®ĻąĶ¬ĪļÖŹµÄĮæµÄ±ūĻ©ŗĻ³ÉÕż¶”Č©Ź±£¬ĪĀ¶ČŌ½øߣ¬Ėł»ńµĆµÄÕż¶”Č©µÄ²śĮæŅ»¶ØŌ½“ó
D£®ĪĀ¶Č²»±äŹ±£¬Ö»øıäĢåĻµµÄŃ¹Ē棬¶Ō²śĀŹĆ»ÓŠÓ°Ļģ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ½ĖÕŹ”øßČż°ŁŠ£“óĮŖæ¼Ņ»Ä£æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
Õż¶”Č©æÉŌŚōŹ»łīÜ“ß»ÆĻĀÓɱūĻ©Ēā¼×õ£»Æ»ńµĆ£ŗCH3CH=CH2+CO+H2 CH3CH2CH2CHO£ØÕż¶”Č©£©ø±·“Ó¦£ŗCH3CH=CH2+CO+H2
CH3CH2CH2CHO£ØÕż¶”Č©£©ø±·“Ó¦£ŗCH3CH=CH2+CO+H2 £ØCH3£©2CHCHO£ØŅģ¶”Č©£©£¬ĪĀ¶Č¶Ō±ūĻ©Ēā¼×õ£»Æ²śĪļÖŠÕż/ŅģČ©±ČµÄÓ°ĻģČēĻĀĶ¼£ŗ
£ØCH3£©2CHCHO£ØŅģ¶”Č©£©£¬ĪĀ¶Č¶Ō±ūĻ©Ēā¼×õ£»Æ²śĪļÖŠÕż/ŅģČ©±ČµÄÓ°ĻģČēĻĀĶ¼£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £Ø £©
| A£®±ūĻ©Ēā¼×õ£»Æ·“Ó¦ŹĒ·ÅČČ·“Ó¦ |
| B£®±ūĻ©ÓėCO4H2»ģŗĻ“ß»ÆŹ±£¬æÉÄÜÓŠ±ūĶé¼°Õż¶”“¼µČø±²śĪļÉś³É |
| C£®ĻąĶ¬ĪļÖŹµÄĮæµÄ±ūĻ©ŗĻ³ÉÕż¶”Č©Ź±£¬ĪĀ¶ČŌ½øߣ¬Ėł»ńµĆµÄÕż¶”Č©µÄ²śĮæŅ»¶ØŌ½“ó |
| D£®ĪĀ¶Č²»±äŹ±£¬Ö»øıäĢåĻµµÄŃ¹Ē棬¶Ō²śĀŹĆ»ÓŠÓ°Ļģ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com