
(8·Ö) ijĪŽÉ«ĶøĆ÷µÄČÜŅŗæÉÄÜŗ¬ÓŠK+”¢Cu2+”¢Ca2+”¢Br”Ŗ”¢SO42”Ŗ”¢CO32”Ŗ”¢Cl”ŖÖŠµÄ¼øÖÖ£¬½ųŠŠČēĻĀŹµŃé£ŗ
£Ø1£©µĪ¼ÓĀČ»Æ±µČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś”£½«³ĮµķĀĖ³ö£¬²¢½«ĀĖŅŗ·ÖĪŖĮ½·Ż£¬²śÉśµÄ³Įµķæɲæ·ÖČÜÓŚĻ”ĻõĖį”£
£Ø2£©Č”Ņ»·ŻĀĖŅŗ£¬ĻņĘäÖŠ¼ÓČėĀČĖ®²¢¼ÓČėĖÄĀČ»ÆĢ¼£¬Õńµ“ŗó¾²ÖĆ£¬ĖÄĀČ»ÆĢ¼²ć³Ź³ČÉ«”£
£Ø3£©ĻņĮķŅ»·ŻĀĖŅŗÖŠ¼ÓČėĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬øĆ³Įµķ²»ČÜÓŚĻ”ĻõĖį”£
£Ø4£©Č”ŌČÜŅŗ×öŃęÉ«ŹµŃ飬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģµ½×ĻÉ«”£
ŹŌÅŠ¶Ļ£ŗøĆČÜŅŗÖŠŅ»¶ØÓŠ Ąė×Ó£¬æĻ¶Øƻӊ Ąė×Ó£¬æÉÄÜÓŠ Ąė×Ó”£ŅŖ½ųŅ»²½Č·¶ØæÉÄÜ“ęŌŚµÄĄė×Ó£¬»¹ŠčŅŖ×öµÄŹµŃéŹĒ
(8·Ö) K+”¢Br”Ŗ”¢SO42”Ŗ”¢CO32”Ŗ£»Cu2+”¢Ca2+£» Cl”Ŗ£»Č”ŌČÜŅŗŹŹĮæÓŚŹŌ¹ÜÖŠ£¬¼ÓČėĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬øĆ³Įµķ²»ČÜÓŚĻ”ĻõĖį”£
½āĪöŹŌĢā·ÖĪö£ŗČÜŅŗŹĒĪŽÉ«µÄ£¬ĖłŅŌŅ»¶ØƻӊCu2+”£øł¾Ż£Ø1£©æÉÖŖ£¬Éś³ÉµÄ°×É«³ĮµķÓ¦øĆŹĒĮņĖį±µŗĶĢ¼Ėį±µµÄ»ģŗĻĪļ£¬ĖłŅŌŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO42”Ŗ”¢CO32”Ŗ£¬Ōņ¾ĶŅ»¶ØƻӊCa2+”£øł¾ŻŹµŃé£Ø2£©æÉÖŖ£¬ĖÄĀČ»ÆĢ¼²ć³Ź³ČÉ«£¬ĖµĆ÷·“Ó¦ÖŠÓŠµ„ÖŹäåÉś³É£¬ĖłŅŌŅ»¶Øŗ¬ÓŠBr”Ŗ”£øł¾ŻŹµŃé£Ø3£©æÉÖŖ£¬°×É«³ĮµķÓ¦øĆŹĒĀČ»ÆŅų£¬µ«ÓÉÓŚÖ®Ē°¼ÓČėĮĖĀČ»Æ±µČÜŅŗ£¬ŹµŃé²»ÄÜČ·¶ØŹĒ·ńŗ¬ÓŠĀČĄė×Ó”£øł¾ŻŹµŃé£Ø4£©æÉÖŖ£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģµ½×ĻÉ«£¬ĖµĆ÷ŗ¬ÓŠ¼ŲĄė×Ó”£¼ģŃéĀČĄė×ӵķ½·ØŹĒČ”ŌČÜŅŗŹŹĮæÓŚŹŌ¹ÜÖŠ£¬¼ÓČėĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬øĆ³Įµķ²»ČÜÓŚĻ”ĻõĖį”£
æ¼µć£ŗæ¼²éĄė×ӵĹ²“ę¼°Ąė×ÓµÄÓŠ¹Ų¼ģŃéµČ
µćĘĄ£ŗ½ųŠŠĪļÖŹµÄ¼ģŃ鏱£¬ŅŖŅĄ¾ŻĪļÖŹµÄĢŲŹāŠŌÖŹŗĶĢŲÕ÷·“Ó¦£¬Ń”ŌńŹŹµ±µÄŹŌ¼ĮŗĶ·½·Ø£¬×¼Č·¹Ū²ģ·“Ó¦ÖŠµÄĆ÷ĻŌĻÖĻó£¬ČēŃÕÉ«µÄ±ä»Æ”¢³ĮµķµÄÉś³ÉŗĶČܽā”¢ĘųĢåµÄ²śÉśŗĶĘųĪ¶”¢»šŃęµÄŃÕÉ«µČ£¬½ųŠŠÅŠ¶Ļ”¢ĶĘĄķ”¢ŃéÖ¤¼“æÉ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ŹµŃé ±ąŗÅ |
ŹµŃéÄæµÄ | T/K | “߻ƼĮÓĆĮæ/g | C/mol?l-1 | |
| KMnO4 | H2C2O4 | ||||
| ¢Ł | ĪŖŅŌĻĀŹµŃé×÷²Īæ¼ | 298 | 0.5 | 0.01 | 0.1 |
| ¢Ś | Ģ½¾æKMnO4ĖįŠŌČÜŅŗµÄÅØ¶Č¶ŌøĆ·“Ó¦ĖŁĀŹµÄÓ°Ļģ | 298 | 0.5 | 0.001 | 0.1 |
| ¢Ū | 323 | 0.5 | 0.01 | 0.1 | |
| ¢Ü | Ģ½¾æ“߻ƼĮ¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ | 0.1 | |||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£Ø17·Ö£©¾ßÓŠ»¹ŌŠŌµÄĪŽĖ®²ŻĖįŹĒĪŽÉ«ĪŽ³ōµÄĶøĆ÷½į¾§»ņ°×É«·ŪÄ©”£²ŻĖįŌŚÅØĮņĖį²¢¼ÓČČĢõ¼žĻĀČŻŅ×ĶŃČ„Ė®·Ö£¬·Ö½āĪŖ¶žŃõ»ÆĢ¼ŗĶŅ»Ńõ»ÆĢ¼”£
£Ø1£© ²ŻĖį£ØH2C2O4£©·Ö½āµÄ»Æѧ·½³ĢŹ½ ĪŖ£ŗ £¬
ĻĀĮŠ×°ÖĆÖŠ£¬æÉÓĆÓŚ²ŻĖį·Ö½āÖĘČ”ĘųĢåµÄŹĒ ”££ØĢī×ÖÄø£©
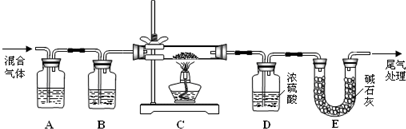
£Ø2£© ijĢ½¾æŠ”×éĄūÓĆ²ŻĖį·Ö½ā²śÉśµÄ»ģŗĻĘųĢåŗĶĢśŠā·“Ó¦Ą“²ā¶ØĢśŠāѳʷ×é³É£Ø¼Ł¶ØĢśŠāÖŠÖ»ÓŠFe2O3”¤nH2OŗĶFeĮ½ÖֳɷŻ£©£¬ŹµŃé×°ÖĆČēĻĀĶ¼ĖłŹ¾£¬Ēė»Ų“š£ŗ
¢Ł ĪŖµĆµ½øÉŌļ”¢“æ¾»µÄCOĘųĢ壬Ļ“ĘųĘæA”¢BÖŠŹ¢·ÅµÄŹŌ¼Į·Ö±šŹĒ ”¢ ”£
¢Ś ŌŚµćČ¼¾Ę¾«µĘÖ®Ē°Ó¦½ųŠŠµÄ²Ł×÷ŹĒ£ŗ£Øa£© £»£Øb£©ĶØČė»ģŗĻĘųĢåŅ»¶ĪŹ±¼ä”£
¢Ū ×¼Č·³ĘĮæѳʷµÄÖŹĮæ10.00 gÖĆÓŚÓ²ÖŹ²£Į§¹ÜÖŠ£¬³ä·Ö·“Ó¦ŗóĄäČ“”¢³ĘĮæ£¬Ó²ÖŹ²£Į§ ¹ÜÖŠŹ£Óą¹ĢĢåÖŹĮæĪŖ8.32 g£¬DÖŠÅØĮņĖįŌöÖŲ0.72 g£¬Ōņn= £Ø¼Ł¶ØFeŗĶH2O ²»·¢Éś·“Ó¦£¬ŹµŃé¹ż³ĢÖŠĆæ²½¾łĶźČ«ĪüŹÕ»ņ·“Ó¦£©”£
¢Ü ŌŚ±¾ŹµŃéÖŠ£¬ĻĀĮŠĒéæö»įŹ¹²ā¶Ø½į¹ūnĘ«“óµÄŹĒ £ØĢī×ÖÄø£©”£
a£®Č±ÉŁĻ“ĘųĘæB b£®Č±ÉŁ×°ÖĆE
c£®·“Ó¦ŗó¹ĢĢåŹĒĢśŗĶÉŁĮæFe2O3 d£®·“Ó¦ŗó¹ĢĢåŹĒĢśŗĶÉŁĮæFe2O3”¤nH2O
£Ø3£© øĆĢ½¾æŠ”×黹ĄūÓĆKMnO4ĖįŠŌČÜŅŗÓėH2C2O4ČÜŅŗ·“Ó¦¹ż³ĢÖŠČÜŅŗ×ĻÉ«ĻūŹ§µÄ·½·Ø£¬ŃŠ¾æÓ°Ļģ·“Ó¦ĖŁĀŹµÄŅņĖŲ”£
¢Ł ĒėĶź³ÉŅŌĻĀŹµŃéÉč¼Ę±ķ£Ø±ķÖŠ²»ŅŖĮōæÕøń£©£ŗ
£ØĆæ“ĪŹµŃéKMnO4ĖįŠŌČÜŅŗµÄÓĆĮæ¾łĪŖ4mL”¢H2C2O4ČÜŅŗµÄÓĆĮæ¾łĪŖ2mL£¬“߻ƼĮ µÄÓĆĮææÉŃ”Ōń0.5g”¢0g£©
| ŹµŃé ±ąŗÅ | ŹµŃéÄæµÄ | T/K | “߻ƼĮÓĆĮæ/g | C/mol”¤l-1:] | |
| KMnO4 | H2C2O4 | ||||
| ¢Ł | ĪŖŅŌĻĀŹµŃé×÷²Īæ¼ | 298 | 0.5 | 0.01 | 0.1 |
| ¢Ś | Ģ½¾æKMnO4ĖįŠŌČÜŅŗµÄÅØ¶Č¶ŌøĆ·“Ó¦ĖŁĀŹµÄÓ°Ļģ | 298 | 0.5 | 0.001 | 0.1 |
| ¢Ū |
| 323 | 0.5 | 0.01 | 0.1 |
| ¢Ü | Ģ½¾æ“߻ƼĮ¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ |
|
|
| 0.1 |
¢Ś ČōŅŖ×¼Č·¼ĘĖć·“Ó¦ĖŁĀŹ£¬øĆŹµŃéÖŠ»¹Šč²ā¶ØČÜŅŗ×ĻÉ«ĻūŹ§ĖłŠčŅŖµÄŹ±¼ä”£ĒėÄćÉč¼Ę³ö
Ķعż²ā¶ØĶŹÉ«Ź±¼ä³¤¶ĢĄ“ÅŠ¶ĻÅØ¶Č“óŠ”Óė·“Ó¦ĖŁĀŹ¹ŲĻµµÄŹµŃé·½°ø ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŌĘÄĻŹ”ÓńĻŖŅ»ÖŠøßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢāŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©(8·Ö) ijĪŽÉ«ĶøĆ÷ČÜŅŗæÉÄÜ“ęŌŚNa£«”¢Fe3£«”¢Ba2£«”¢NO3£”¢CO32£”¢HCO3£”¢SO42£ÖŠµÄ¼øÖÖĄė×Ó£¬ĻÖÓŠČēĻĀ²Ł×÷£ŗ
¢ń”¢Č”ŹŹĮæøĆČÜŅŗ¼ÓČėCaCl2ČÜŅŗĪŽ³Įµķ£¬¼ĢŠųµĪ¼ÓŃĪĖį²śÉśĪŽÉ«ĪŽĪ¶µÄĘųĢ唣
¢ņ”¢ĮķČ”øĆČÜŅŗµĪ¼ÓŅ»¶ØĮæµÄNaOHČÜŅŗÓŠ°×É«³ĮµķÉś³É”£
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł øĆČÜŅŗÖŠŅ»¶Ø“ęŌŚµÄĄė×ÓÓŠ__________£¬Ņ»¶Ø²»“ęŌŚµÄĄė×Ó__________”£
¢Ś ÓĆŅ»øöĄė×Ó·½³ĢŹ½±ķŹ¾²Ł×÷¢ŚµÄŹµŃéĻÖĻó£ŗ
______________________________________________________ӣ
£Ø2£©£Ø4·Ö£©ĻĀĮŠø÷ĻīÖŠµÄĮ½ÖÖĪļÖŹŌŚČÜŅŗÖŠµÄ·“Ó¦£¬æÉÓĆĶ¬Ņ»Ąė×Ó·½³ĢŹ½±ķŹ¾µÄŹĒ£Ø £©£¬ĒėŠ“³öøĆĄė×Ó·“Ó¦·½³ĢŹ½£ŗ___________________________ __________________”£
__________________ӣ
| A£®ŃĪĖįŗĶÉÕ¼īČÜŅŗ”¢ĮņĖįŗĶĒāŃõ»Æ±µČÜŅŗ |
B£®“æ ¼īČÜŅŗŗĶŃĪĖį”¢ŹÆ»ŅŹÆŗĶĮņĖį ¼īČÜŅŗŗĶŃĪĖį”¢ŹÆ»ŅŹÆŗĶĮņĖį |
| C£®ĀČ»Æ±µČÜŅŗŗĶĮņĖįÄĘČÜŅŗ”¢ĻõĖį±µČÜŅŗŗĶĮņĖį |
| D£®Ģ¼Ėįļ§ČÜŅŗŗĶĒāŃõ»ÆÄĘČÜŅŗ”¢Ģ¼Ėįļ§ČÜŅŗŗĶĒāŃõ»ÆøĘČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģ½Ī÷Ź”øßŅ»µŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(8·Ö) ijĪŽÉ«ĶøĆ÷µÄČÜŅŗæÉÄÜŗ¬ÓŠK+”¢Cu2+”¢Ca2+”¢Br”Ŗ”¢SO42”Ŗ”¢CO32”Ŗ”¢Cl”ŖÖŠµÄ¼øÖÖ£¬½ųŠŠČēĻĀŹµŃé£ŗ
£Ø1£©µĪ¼ÓĀČ»Æ±µČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś”£½«³ĮµķĀĖ³ö£¬²¢½«ĀĖŅŗ·ÖĪŖĮ½·Ż£¬²śÉśµÄ³Įµķæɲæ·ÖČÜÓŚĻ”ĻõĖį”£
£Ø2£©Č”Ņ»·ŻĀĖŅŗ£¬ĻņĘäÖŠ¼ÓČėĀČĖ®²¢¼ÓČėĖÄĀČ»ÆĢ¼£¬Õńµ“ŗó¾²ÖĆ£¬ĖÄĀČ»ÆĢ¼²ć³Ź³ČÉ«”£
£Ø3£©ĻņĮķŅ»·ŻĀĖŅŗÖŠ¼ÓČėĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬øĆ³Įµķ²»ČÜÓŚĻ”ĻõĖį”£
£Ø4£©Č”ŌČÜŅŗ×öŃęÉ«ŹµŃ飬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģµ½×ĻÉ«”£
ŹŌÅŠ¶Ļ£ŗøĆČÜŅŗÖŠŅ»¶ØÓŠ Ąė×Ó£¬æĻ¶Øƻӊ Ąė×Ó£¬æÉÄÜÓŠ Ąė×Ó”£ŅŖ½ųŅ»²½Č·¶ØæÉÄÜ“ęŌŚµÄĄė×Ó£¬»¹ŠčŅŖ×öµÄŹµŃéŹĒ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com