
(16��)ij��Ȼ��Ļ�ѧ��ɿ���ΪaNa2CO3��bNaHCO3��cH2O(a��b��cΪ������)��Ϊȷ������ɣ���ѧ��ȤС���ͬѧ����������ʵ�飺
(1)���Է���
��ȡ������Ȼ����Ʒ�����Թ��У��þƾ��Ƽ��ȣ����Թܿ���Һ�����ɣ���Һ����ʹ��ˮ����ͭ�������ܷ�˵����Ʒ�к��ᾧˮ���Լ������ɡ�
���������һ����ʵ�鷽����ȷ����Ʒ�к���CO32�����ӡ�
(2)��������
��С��ͬѧ�������ͼ��ʾװ�ã��ⶨ��Ȼ��Ļ�ѧ��ɡ�
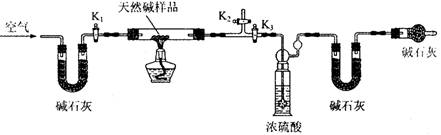
A B C D E
ʵ�鲽�裺
�ٰ���ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ����� ��
A����ʯ�ҵ������� ��
�ڳ�ȡ��Ȼ����Ʒ7.3g�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿ������Ϊ 87.6g��װ��ʯ�ҵ�U��D������Ϊ74.7g��
�۴���K1��K2���ر�K3������������������ӡ�
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��ȣ������ٲ�������Ϊֹ��
�ݴ���K1������������������ӣ�Ȼ��Ƶ�װŨ�����ϴ��ƿ����Ϊ88.5g��װ��ʯ�ҵ�U��D������Ϊ75.8g���ò����л���������������ӵ�Ŀ����
��
�����Ƶ���
����Ȼ��Ļ�ѧʽΪ ��
(1)���Է���
�ٲ���˵������Ϊ��Ȼ����Ʒ�еġ�NaHC03�����ȷֽ�Ҳ�ɲ���ˮ��
��ȡ������Ȼ����Ʒ����ˮ������������CaCl2��Һ(��BaCl2��Һ)�����˲�ϴ�ӳ�����������м���ϡ���ᣬ������������ͨ������ʯ��ˮ�С����л�������˵����Ȼ����Ʒ�к���CO32-���ӡ���ÿС��3�֣���6�֣�
(2)�������� ��ÿ��2�֣���6�֣�
ʵ�鲽�裺
�ټ��װ�õ������� ��ȥͨ������е�CO2��H2O
��ʹ��Ӧ���ɵ�CO2��H2O���������
�����Ƶ���
Na2CO3��2NaHCO3��H2O (4��)
����������1����������Ȼ����Ʒ�еġ�NaHC03�����ȷֽ�Ҳ�ɲ���ˮ�����Բ���˵����
��Ҫ����CO32���������ó���������ȡ������Ȼ����Ʒ����ˮ������������CaCl2��Һ(��BaCl2��Һ)�����˲�ϴ�ӳ�����������м���ϡ���ᣬ������������ͨ������ʯ��ˮ�С����л�������˵����Ȼ����Ʒ�к���CO32-���ӡ�
��2����װ�����Ӻú�����Ҫ����װ�õ������ԡ����ڿ����к���ˮ������CO2�������ʵ�飬���Լ�ʯ�ҵ������dz�ȥͨ������е�CO2��H2O��
�����ڷ�Ӧ�в���������������װ���У�����ͨ�������������ʹ��Ӧ���ɵ�CO2��H2O��������ա�
��Ũ����ͼ�ʯ�ҷֱ����ӵ�������0.9g��1.1g������ˮ��CO2�����ʵ����ֱ���0.05mol��0.025mol������Ϊ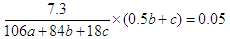 ��
��
���a�Ub�Uc��1�U2�U1�����Ի�ѧʽΪNa2CO3��2NaHCO3��H2O��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013������ʡ������ѧ�¸�����ѧ��Ӧ�Բ��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(16��)ij��Ȼ��Ļ�ѧ��ɿ���ΪaNa2CO3��bNaHCO3��cH2O(a��b��cΪ������)��Ϊȷ������ɣ���ѧ��ȤС���ͬѧ����������ʵ�飺
(1)���Է���
��ȡ������Ȼ����Ʒ�����Թ��У��þƾ��Ƽ��ȣ����Թܿ���Һ�����ɣ���Һ����ʹ��ˮ����ͭ�������ܷ�˵����Ʒ�к��ᾧˮ���Լ������ɡ�
���������һ����ʵ�鷽����ȷ����Ʒ�к���CO32�����ӡ�
(2)��������
��С��ͬѧ�������ͼ��ʾװ�ã��ⶨ��Ȼ��Ļ�ѧ��ɡ�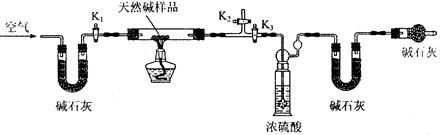
A B C D E
ʵ�鲽�裺
�ٰ���ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ����� ��
A����ʯ�ҵ������� ��
�ڳ�ȡ��Ȼ����Ʒ7.3g�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿ������Ϊ 87.6g��װ��ʯ�ҵ�U��D������Ϊ74.7g��
�۴���K1��K2���ر�K3������������������ӡ�
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��ȣ������ٲ�������Ϊֹ��
�ݴ���K1������������������ӣ�Ȼ��Ƶ�װŨ�����ϴ��ƿ����Ϊ88.5g��װ��ʯ�ҵ�U��D������Ϊ75.8g���ò����л���������������ӵ�Ŀ����
��
�����Ƶ���
����Ȼ��Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com