
·ÖĪö £Ø1£©HCOONaČÜŅŗĖ®½ā³Ź¼īŠŌ£»
£Ø2£©½«0.2mol•L-1µÄHCOOHŗĶ0.1mol•L-1µÄNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬·“Ó¦ŗóÉś³ÉµČĪļÖŹµÄĮæµÄHCOOHŗĶHCOONa£¬øł¾Ż»ģŗĻČÜŅŗµÄĖį¼īŠŌÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©HCOONaČÜŅŗĖ®½ā³Ź¼īŠŌ£¬Ė®½ā·½³ĢŹ½ĪŖ£ŗHCOO-+H2O=HCOOH+OH-£»
¹Ź“š°øĪŖ£ŗHCOO-+H2O=HCOOH+OH-£»
£Ø2£©ĖįµÄĪļÖŹµÄĮæŹĒ¼īµÄĪļÖŹµÄĮæµÄ2±¶£¬ĒŅĖį¼ī¶¼ŹĒŅ»ŌŖµÄ£¬·“Ó¦ŗóÉś³ÉµČĪļÖŹµÄĮæµÄHCOOHŗĶHCOONa£»HCOOHµēĮ÷³öĒāĄė×Ó£¬HCOONaĖ®½āÉś³ÉĒāŃõøłĄė×Ó£¬ČÜŅŗĻŌĖįŠŌ£¬ĖµĆ÷ŅŌµēĄėĪŖÖ÷£¬¼“HCOOHµÄµēĄė³Ģ¶Č“óÓŚHCOONaµÄĖ®½ā³Ģ¶Č£»HCOOHŗĶHCOONaŗĶ“×Ėį¶¼µēĄėÉś³É“×Ėįøł£¬ĒŅµēĄė“óÓŚĖ®½ā£¬¹ŹøĆ»ģŗĻČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ£ŗC£ØHCOO-£©£¾C£ØNa+£©£¾C£ØH+£©£¾C£ØOH-£©
¹Ź“š°øĪŖ£ŗ“óÓŚ£»C£ØHCOO-£©£¾C£ØNa+£©£¾C£ØH+£©£¾C£ØOH-£©£®
µćĘĄ ±¾Ģāæ¼²éĮĖŃĪµÄĖ®½āŌĄķ¼°Ó¦ÓĆ”¢ĖįŹ½ŃĪµÄĖįøłĄė×ӵĵēĄėÓėĖ®½ā³Ģ¶ČµÄ±Č½ĻµČ£¬ĢāÄæÄŃ¶Č²»“󣬲ąÖŲÓŚ»ł“”ÖŖŹ¶µÄ漲飬עŅā°ŃĪÕŃĪµÄĖ®½āĄė×Ó·½³ĢŹ½µÄŹéŠ“£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆĶŠÅĢĢģĘ½³ĘČ”11.7gNaCl | |
| B£® | ÓĆ50mLĮæĶ²ĮæČ”21.48mLĻ”ĮņĖį | |
| C£® | ÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”25.03mLH2SO4ČÜŅŗ | |
| D£® | ÓĆpHŹŌÖ½²ā¶ØHNO3ČÜŅŗµÄpH=3.7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹¤ŅµÖĘŃõ”¢øÖĢśÉśŠā | B£® | ŹÆĄÆČŪ»Æ”¢øɱłÉż»Ŗ | ||
| C£® | ĮøŹ³Äš¾Ę”¢ŃĢ»ØČ¼·Å | D£® | ĘūÓĶ»Ó·¢”¢ŌĘĻūĪķÉ¢ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SO2Ź¹Ę·ŗģČÜŅŗĶŹÉ« | B£® | Ė«ŃõĖ®Ź¹ÓŠÉ«²¼ĢõĶŹÉ« | ||
| C£® | »īŠŌĢæŹ¹ŗģÄ«Ė®ĶŹÉ« | D£® | O3Ź¹Ä³Š©Č¾ĮĻĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĻ© | B£® | ±ūČ² | C£® | ¶”¶žĻ© | D£® | ¼×Ķé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 0.10mol/L Ag+ | B£® | 0.20mol/L Zn2+ | C£® | 0.20mol/L Cu2+ | D£® | 0.20mol/L Pb2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
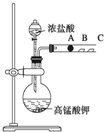 Ä³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼ĘĮĖŅ»×鏵Ń饓Ģ½¾æŌŖĖŲÖÜĘŚĀÉ£®¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼×°ÖĆĄ“ŃéÖ¤Ā±×åŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉ£®A”¢B”¢CČż“¦·Ö±šŹĒÕ“ÓŠNaBrČÜŅŗµÄĆŽ»Ø”¢ŹŖČóµÄµķ·ŪKIŹŌÖ½”¢ŹŖČóŗģÖ½£®ŅŃÖŖ³£ĪĀĻĀÅØŃĪĖįÓėøßĆĢĖį¼ŲÄÜ·“Ӧɜ³ÉĀČĘų£®
Ä³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼ĘĮĖŅ»×鏵Ń饓Ģ½¾æŌŖĖŲÖÜĘŚĀÉ£®¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼×°ÖĆĄ“ŃéÖ¤Ā±×åŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉ£®A”¢B”¢CČż“¦·Ö±šŹĒÕ“ÓŠNaBrČÜŅŗµÄĆŽ»Ø”¢ŹŖČóµÄµķ·ŪKIŹŌÖ½”¢ŹŖČóŗģÖ½£®ŅŃÖŖ³£ĪĀĻĀÅØŃĪĖįÓėøßĆĢĖį¼ŲÄÜ·“Ӧɜ³ÉĀČĘų£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com