
X”¢Y”¢Z”¢W”¢RŹĒ5ÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ĘäŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£XŹĒÖÜĘŚ±ķÖŠŌ×Ó°ė¾¶×īŠ”µÄŌŖĖŲ£¬YŌ×Ó×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹżµÄ3±¶£¬Z”¢W”¢R“¦ÓŚĶ¬Ņ»ÖÜĘŚ£¬RÓėY“¦ÓŚĶ¬Ņ»Ö÷×壬Z”¢WŌ×ÓµÄŗĖĶāµē×ÓŹżÖ®ŗĶÓėY”¢RŌ×ÓµÄŗĖĶāµē×ÓŹżÖ®ŗĶĻąµČ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®ŌŖĖŲY”¢Z”¢W¾ßÓŠĻąĶ¬µē×Ó²ć½į¹¹µÄĄė×Ó£¬Ęä°ė¾¶ŅĄ“ĪŌö“ó
B£®ŌŖĖŲX²»ÄÜÓėŌŖĖŲYŠĪ³É»ÆŗĻĪļX2Y2
C£®ŌŖĖŲY”¢R·Ö±šÓėŌŖĖŲXŠĪ³ÉµÄ»ÆŗĻĪļµÄČČĪČ¶ØŠŌ£ŗXmY£¾XmR
D£®ŌŖĖŲW”¢RµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļ¶¼ŹĒĒæĖį
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
·ĄÖ¹øÖĢśµÄøÆŹ“ŹĒŹĄ½ē¼¶ÄŃĢā£¬ĆæğȫŹĄ½ēøÖ²śĮæµÄĖÄ·ÖÖ®Ņ»ŅņøÆŹ“¶ųĖšŹ§£¬øł¾ŻĻĀĶ¼»Ų“š£ŗ

(1)øÖĢśøÆŹ“Ö÷ŅŖŹĒĪüŃõøÆŹ“£¬øĆøÆŹ“¹ż³ĢÖŠµÄµē¼«·“Ó¦Ź½ĪŖ________________________________________________________________”£
(2)ĪŖ½µµĶijĖ®æāµÄĢśÕ¢Ćŵı»øÆŹ“ĖŁĀŹ£¬æÉŅŌ²ÉÓĆĶ¼¼×·½°ø£¬ĘäÖŠŗø½ÓŌŚĢśÕ¢ĆÅÉĻµÄ¹ĢĢå²ÄĮĻRæÉŅŌ²ÉÓĆ__________”£
A£®Ķ B£®ÄĘ C£®Šæ D£®ŹÆÄ«
(3)Ķ¼ŅŅĖłŹ¾·½°øŅ²æɽµµĶĢśÕ¢Ćŵı»øÆŹ“ĖŁĀŹ£¬ĘäÖŠĢśÕ¢ĆÅÓ¦øĆĮ¬½ÓŌŚÖ±Į÷µēŌ“µÄ________¼«”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠ»śĪļAµÄ½į¹¹¼ņŹ½ČēÓŅĶ¼ĖłŹ¾£¬ĻĀĮŠÓŠ¹ŲøĆĪļÖŹµÄĖµ·ØÕżČ·µÄŹĒ£Ø £©
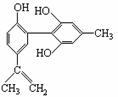
A£®ÓöFeCl3ČÜŅŗĻŌ×ĻÉ«£¬ŅņĪŖøĆĪļÖŹÓė±½·ÓŹōÓŚĶ¬ĻµĪļ
B£®µĪČėĖįŠŌKMnO4ČÜŅŗ£¬¹Ū²ģ×ĻÉ«ĶŹČ„£¬ÄÜÖ¤Ć÷½į¹¹ÖŠ“ęŌŚĢ¼Ģ¼Ė«¼ü
C£®1 moløĆĪļÖŹ·Ö±šÓėÅØäåĖ®ŗĶH2·“Ó¦Ź±£¬×ī¶ąĻūŗÄBr2 ŗĶH2·Ö±šĪŖ4 mol”¢7 mol
D£®øĆ·Ö×ÓÖŠµÄĖłÓŠĢ¼Ō×Ó²»æÉÄܹ²Ę½Ćę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĪåÖÖĢž£ŗ¢Ł2”Ŗ¼×»ł¶”Ķ飻¢Ś2£¬2”Ŗ¶ž¼×»ł±ūĶ飻¢ŪĪģĶ飻¢Ü±ūĶ飻¢Ż¶”Ķ飻
°“ĖüĆĒµÄ·ŠµćÓÉøßµ½µĶµÄĖ³ŠņÅÅĮŠÕżČ·µÄŹĒ
A£®¢Ł>¢Ś>¢Ū>¢Ü>¢Ż B£®¢Ś>¢Ū>¢Ż>¢Ü>¢Ł
C£®¢Ū>¢Ł>¢Ś>¢Ż>¢Ü D£®¢Ü>¢Ż>¢Ś>¢Ł>¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄÜŅ»“Ī¼ų±šŅŅĖį”¢ŅŅ“¼”¢±½”¢Ļõ»ł±½ĖÄÖÖĪļÖŹµÄŹŌÖ½»ņŹŌ¼ĮŹĒ
A£®H2O B£®Na2CO3ČÜŅŗ C£®pHŹŌÖ½ D£®ŹÆČļŹŌÖ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
äå»Æµā(IBr)µÄ»ÆѧŠŌÖŹÓėĀ±ĖŲµ„ÖŹĻąĖĘ£¬ÄÜÓė“󶹏ż½šŹō·“Ӧɜ³É½šŹōĀ±»ÆĪļ£¬ŗĶijŠ©·Ē½šŹōµ„ÖŹ·“Ӧɜ³ÉĻąÓ¦µÄĀ±»ÆĪļ£¬øśĖ®·“Ó¦µÄ·½³ĢŹ½ĪŖIBr£«H2O===HBr£«HIO£¬ĻĀĮŠÓŠ¹ŲIBrµÄŠšŹöÖŠ£¬²»ÕżČ·µÄŹĒ (””””)”£
A£®IBrŹĒĖ«Ō×Ó·Ö×Ó
B£®ŌŚŗܶą·“Ó¦ÖŠ£¬IBrŹĒĒæŃõ»Æ¼Į
C£®ŗĶNaOHČÜŅŗ·“Ӧɜ³ÉNaBrŗĶNaIO
D£®ŗĶĖ®·“Ó¦Ź±£¬¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĀČĘųŹĒĀČ¼ī¹¤ŅµµÄÖ÷ŅŖ²śĘ·Ö®Ņ»£¬ŹĒŅ»ÖÖ³£ÓƵÄĻū¶¾¼Į£¬ĘäĻū¶¾ŌĄķŹĒÓėĖ®·“Ӧɜ³ÉĮĖ“ĪĀČĖį£ŗ
Cl2£«H2OHCl£«HClO
“ĪĀČĖįµÄĒæŃõ»ÆŠŌÄÜɱĖĄĖ®ÖŠµÄ²”¾ś(²»Ö±½ÓÓĆ“ĪĀČĖįĪŖ×ŌĄ“Ė®Ļū¶¾ŹĒŅņĪŖ“ĪĀČĖįŅ×·Ö½ā£¬ĒŅ¶¾ŠŌ½Ļ“ó)”£µ«ŹĒÓÉÓŚĀČĘųÖüŌĖ²»·½±ć£¬ĒŅ¾ßÓŠŅ»¶ØµÄĪ£ĻÕŠŌ£¬ÄæĒ°ÕżÖš½„±»ĘäĖūŠŌÄÜÓÅŌ½µÄĻū¶¾²śĘ·ĖłĢę“ś”£Ēė»Ų“š£ŗ
(1)ĀČ¼ī¹¤ŅµÉś²śĀČĘųµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________ _____________________________________________”£
(2)84Ļū¶¾ŅŗÓėĀČĘųĻą±Č¾ßÓŠÖüŌĖ·½±ćµČÓÅµć£¬ÓĆĀČĘųÓėÉÕ¼īČÜŅŗ·“Ó¦Öʱø84Ļū¶¾ŅŗµÄĄė×Ó·½³ĢŹ½ĪŖ__________________________________________ _______________________________________________________________”£
(3)¶žŃõ»ÆĀČ(ClO2)ŹĒÄæĒ°¹ś¼ŹÉĻ¹«ČĻµÄ×īŠĀŅ»“śµÄøߊ§”¢¹ćĘ×”¢°²Č«µÄɱ¾ś”¢±£ĻŹ¼Į”£ĪŅ¹śæĘѧ¼ŅŃŠ·¢ĮĖÓĆĀČĘųŃõ»ÆŃĒĀČĖįÄĘ(NaClO2)¹ĢĢåÖʱø¶žŃõ»ÆĀȵķ½·Ø£¬Ęä»Æѧ·½³ĢŹ½ĪŖ_________________________________________ _______________________________________________________________________________________________________”£
(4)Ņ»Ī»Ķ¬Ń§Éč¼ĘĮĖŅ»Ģ×ÓĆÅØŃĪĖįŗĶKMnO4¹ĢĢåÖĘȔɣĮæĀČĘų²¢±Č½ĻĀČĘųÓėµāµ„ÖŹµÄŃõ»ÆŠŌĒæČõµÄĪ¢ŠĶ×°ÖĆ(ČēĶ¼ĖłŹ¾)”£

¢ŁĻĀĮŠČÜŅŗÄÜĪüŹÕCl2µÄŹĒ____________________________”£
A£®±„ŗĶŹ³ŃĪĖ® B£®±„ŗĶNa2SO3ČÜŅŗ
C£®±„ŗĶNaOHČÜŅŗ D£®ÅØĮņĖį
¢ŚÄÜĖµĆ÷Cl2µÄŃõ»ÆŠŌĒæÓŚI2µÄŹµŃéĻÖĻóŹĒ____________________________ ____________________________________________________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹ŲÓŚ¹č¼°Ęä»ÆŗĻĪļµÄŠšŹöÖŠ£¬“ķĪóµÄŹĒ (””””)”£
A£®¹čŹĒĮ¼ŗƵİėµ¼Ģå²ÄĮĻ
B£®¶žŃõ»Æ¹čÄÜÓėŹÆ»ŅŹÆ·“Ó¦£ŗSiO2£«CaCO3 CaSiO3£«CO2”ü
CaSiO3£«CO2”ü
C£®æÉŅŌÓĆ½¹Ģ滹Ō¶žŃõ»Æ¹čÉś²ś¹č£ŗSiO2£«2C Si£«2CO”ü
Si£«2CO”ü
D£®Ė®ÄąµÄÖ÷ŅŖ³É·ÖŹĒNa2SiO3”¢CaSiO3ŗĶSiO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼Į½Ō²Ļą½»²æ·ÖA”¢B”¢C”¢D·Ö±š±ķŹ¾Į½ĪļÖŹ¼äµÄ·“Ó¦”£ĻĀĮŠø÷¶ŌÓ¦·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹéŠ“²»ÕżČ·µÄŹĒ (””””)”£
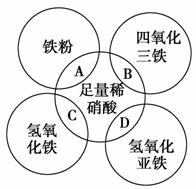
A£®Fe£«4H£«£«NO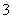 ===Fe3£«£«NO”ü£«2H2O
===Fe3£«£«NO”ü£«2H2O
B£®Fe3O4£«8H£«===Fe2£«£«2Fe3£«£«4H2O
C£®Fe(OH)3£«3H£«===Fe3£«£«3H2O
D£®3Fe(OH)2£«10H£«£«NO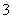 ===3Fe3£«£«NO”ü£«8H2O
===3Fe3£«£«NO”ü£«8H2O
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com