



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
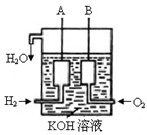
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O(g) = H2(g)��1/2 O2(g)��H��+286kJ��mol-1 |
| B��2H2(g)+ O2(g)=2H2O(l)��H����572kJ��mol-1 |
| C��H2(g)+ 1/2O2(g)= H2O(g)��H����286kJ��mol-1 |
| D��H2(g)��1/2 O2(g)=H2O(l)��H��+286 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
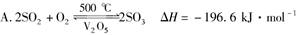
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��g��+1/2O
��g��+1/2O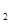 ��g����CO��g��+2H
��g����CO��g��+2H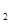 ��g��
��g�� 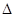 H1����35.6kJ��mol
H1����35.6kJ��mol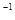
 ��g��+2O
��g��+2O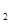 ��g����CO2��g��+2H2O��g��
��g����CO2��g��+2H2O��g�� 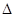 H2����890.3kJ��mol
H2����890.3kJ��mol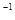
 ��g��+CO
��g��+CO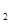 ��g����2CO��g��+2H
��g����2CO��g��+2H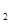 ��g��
��g�� 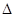 H3��247.3kJ��mol
H3��247.3kJ��mol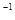
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c(NH3)/( mol ��/L-1) | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |

 �����ʵ���Ũ�� ��
�����ʵ���Ũ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
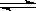 2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����
2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����| A�� Q2=2Q1 | B�� Q2��2Q1 | C�� Q2��Q1 | D��Q2��Q1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȷ�Ӧ | B�����ȷ�Ӧ | C���ų�832 kJ���� | D������183 kJ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com