
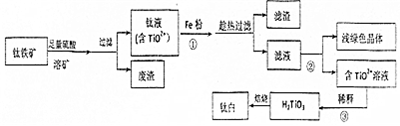
���� I����������Ҫ�ɷ�FeTiO3��������Ϊ�����ۣ�Ϊԭ�ϣ�����ϡ�����ܽ����ˮ�н�ȡ����ȡҺ����Fe2+�����˵õ���Һ����TiOSO4 ��FeSO4�����̢��м���Fe���Է�ֹ�������ӱ����������ȹ��ˣ���Һ����Ũ������ȴ�ᾧ������ϴ�ӵõ�FeSO4•7H2O������Һ��ҪTiOSO4������ˮˮ���������ƫ����H2TiO3��TiO2•H2O�������յõ�TiO2��
��1������������ᷴӦ���Ƶ��������ѣ�����������ˮ�����ԭ���غ���ƽ��д�õ���ѧ����ʽ������Ӵ����������ܿ�Ч�ʣ�
��2�������ᷴӦ�����������ӡ����������ۻ�ԭ������Ϊ�������ӣ���ֹ�ѻ�ԭ��Fe2+���±�����������
��3�������ڵõ�dz��ɫ���壬Ϊ�ᾧˮ������ˮ������������Ϊ��ֹ�ᾧˮ���ȷֽ⣬����ȴ�ᾧ���ᾧ����ˣ�
��4��TiO2+ˮ������H2TiO3��H+���ٽ�H2TiO3�����ɣ���ʹˮ��ƽ�������ƶ�����ͨ�����Ȼ��ˮʵ�֣�
II����5������һ�����ʵ���Ũ�ȵ���Һ����������Ϊ�����㡢��������ȡ�����ܽ⣨ϡ�ͣ�����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��ݴ��ж���Ҫ��������
��6����KSCN��ָʾ�����յ�ʱNH4Fe��SO4��2������Ti3+��Ӧ������Ѫ��ɫ��Fe��SCN��3��
��7�����ݵ�ʧ�����غ㣺1Ti3+��1Fe3+����n��Fe3+��=n��Ti3+��=n��TiO2��=cV��10-3mol���Դ˽��м��㣻
��8��A���յ�ʱ���������ӵζ�����Һ�棬�������ƫС��
B���ζ��õ���ƿ�ڵζ�ǰ����������ˮ����Ӱ�죻
C������Fe3+����Һʱ���ձ��е���Һ����������һЩ������ƫС���ζ���Һ�е�Ti3+��Fe3+ƫ�࣮
��� �⣺I����1������������ᷴӦ���Ƶ��������ѣ�����������ˮ�����ԭ���غ���ƽ��д�õ���ѧ����ʽ��FeTiO3+2H2SO4=TiOSO4+FeSO4+2H2O��Ϊ����ܿ�Ч�ʣ��ɲ�ȡ����ʯ���飬������Ӵ������
�ʴ�Ϊ��FeTiO3+2H2SO4=TiOSO4+FeSO4+2H2O������ʯ���飻
��2�������ᷴӦ�����������ӡ����������ӷ�ӦΪ��Fe+2H+�TFe2++H2�������������ױ�������Fe3+�����ۻ�ԭ������Ϊ�������ӣ�Fe+2Fe3+=3Fe2+����ֹ�ѻ�ԭ��Fe2+���±�������������֤Fe3+ȫ��ת��ΪFe2+��
�ʴ�Ϊ��Fe+2H+�TFe2++H2����Fe+2Fe3+=3Fe2+��
��3���ڢٲ������ۻ�ԭFe3+����ֹ�ѻ�ԭ��Fe2+���±��������������˳�ȥ������Ϊ��ֹ�ᾧˮ���ȷֽ⣬�ڢڲ���ȴ�ᾧ�����˵õ�FeSO4•7H2O���˷���δֱ��ϡ����Һ���H2TiO3��ͬʱ���FeSO4•7H2O��
�ʴ�Ϊ����ȴ�ᾧ�����ˣ�ͬʱ���FeSO4•7H2O��
��4��TiO2+ˮ������H2TiO3�����ӷ���ʽ��TiO2++2H2O=H2TiO3��+2H+��ˮ�����ȣ����ȿɴٽ�H2TiO3�����ɣ��ӹ���ˮҲ�ɴٽ�H2TiO3�����ɣ�
�ʴ�Ϊ��TiO2++2H2O=H2TiO3��+2H+�����Ȼ�ӹ���ˮ��
II����5������һ�����ʵ���Ũ�ȵ���Һ����������Ϊ�����㡢��������ȡ�����ܽ⣨ϡ�ͣ�����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ����Ͳ�����ձ�������������ͷ�ιܡ�����ƿ�ȣ�����100mL����Һ��100mL����ƿ��
�ʴ�Ϊ��100mL����ƿ��
��6�����ﵽ�ζ��յ�ʱ���ټ���NH4Fe��SO4��2����Һ��Fe3+����������Ѫ��ɫ��Fe��SCN��3���Ұ���Ӳ���ɫ��
�ʴ�Ϊ��KSCN��Һ��
��7�����ݵ�ʧ�����غ㣬�У�1Ti3+��1Fe3+����n��Fe3+��=n��Ti3+��=n��TiO2��=cV��10-3mol������������Ϊ$\frac{cVM}{1000W}$��$\frac{cVM}{10W}$%��
�ʴ�Ϊ��$\frac{cVM}{1000W}$��$\frac{cVM}{10W}$%��
��8��A���յ�ʱ���������ӵζ�����Һ�棬�������ƫС��TiO2������ƫ�ͣ���A����
B���ζ��õ���ƿ�ڵζ�ǰ����������ˮ������Һ�����ʵ������䣬��Ӱ�죬��B����
C������Fe3+����Һʱ���ձ��е���Һ����������һЩ���������Ƶ���Һ�����ʵ����ʵ���ƫС������Fe3+��Һƫ�ͣ�TiO2������ƫ�ߣ���C��ȷ��
�ʴ�Ϊ��C��
���� ���⿼�����Ѱ��Ʊ��������������ʺͷ�Ӧ�����ǽ���ؼ�������ѧ������ѧ֪ʶ���ۺ�Ӧ��������ע�����ʷ�������̷����жϣ��������ʵ�Ӧ�ã���Ŀ�Ѷ��еȣ�


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
| NaOH��Һ | ������Һ | ����Cu��OH��2 | ������ | |
| X | �кͷ�Ӧ | ������ | �ܽ� | �������� |
| Y | ������ | ������ | ���Ⱥ���ש��ɫ���� | �������� |
| Z | ˮ�ⷴӦ | ������ | ���Ⱥ���ש��ɫ���� | ������ |
| W | ˮ�ⷴӦ | ������ | ������ | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
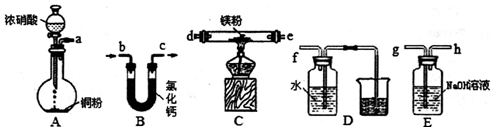
 ����ͼ��ʾװ�ó�ȥ��CN-��CI-��ˮ�е�CN-ʱ��������ҺpHΪ9-10������������ClO-��CN-����Ϊ��������Ⱦ�����壬����˵������ȷ���ǣ�������
����ͼ��ʾװ�ó�ȥ��CN-��CI-��ˮ�е�CN-ʱ��������ҺpHΪ9-10������������ClO-��CN-����Ϊ��������Ⱦ�����壬����˵������ȷ���ǣ�������| A�� | ��ʯī���������������� | |
| B�� | �����ĵ缫��ӦʽΪ��Cl-+2OH--2e-�TClO-+H2O | |
| C�� | �����ĵ缫��ӦʽΪ��2H++2e-=H2�� | |
| D�� | ��ȥCN-�ķ�Ӧ��2CN-+5ClO-+2H+�TN2��+2CO2��+5Cl-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ж��зǽ���Ԫ�� | |
| B�� | ͬ����Ԫ���У���A��Ԫ�ص�ԭ�Ӱ뾶��� | |
| C�� | ��A��Ԫ�ص�ԭ�ӣ���뾶Խ��Խ���õ����� | |
| D�� | Ԫ�����ڱ��дӢ�B����B 10�����е�Ԫ�ض��ǽ���Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������KMnO4��Һ���Լ����̷���FeSO4•7H2O���Ƿ���� | |
| B�� | �Ǽ��Լ�ֻ������˫ԭ�ӵĵ��ʷ��ӣ���Cl2���� | |
| C�� | �ڹ��ۻ���������ڣ����ܴ������Ӽ� | |
| D�� | �ڵ�ԭ���У�������Ϊ7����������һ��Ϊ7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���칤����С���ʯ�ң��������Ϊ�á���ġ�ʯ�ҡ�ָ����Ca��OH��2 | |
| B�� | ���ư�ʮ�����С�����Ϳ����������ͭ�����䡰���ࡱ�ǿ�����ͭ�� | |
| C�� | �����ݸ�Ŀ���С�������������н��֮�ң������Ի���֭��ȡ����¡��еļ���K2CO3 | |
| D�� | �����顷�С���ū�����ˮ��ȼ��������ġ��ˮ��ָ����ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ﲻһ���Ǽ��������� | |
| B�� | HC1��H2S��NH3���ǵ���� | |
| C�� | ǿ��ǿ������ӻ����� | |
| D�� | FeBr3��FeCl2��CuS������ֱ���û��Ϸ�Ӧ�Ʊ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com