
����Ŀ��������������ѧ��������ش��������⡣
(1)�����еĵ�ζƷ������(��Ҫ�ɷ��Ȼ���)���ڼ���(��Ҫ�ɷ�̼����)���۰״�(��Ҫ�ɷ�����)�����������ʷֱ�����ˮ����ˮ��Һ���м��Ե���_______________(����ţ���ͬ)����Ҫ�ɷ��������������___________������ܷ�����Ӧ����____________________��
(2)ʳ�β�����������Ȼ���Ļ����ϣ��۲쵽������______________���ñ仯��Ϊ________��Ӧ��
(3)մ��ˮ�����������ڸ��»����ϻᷢ�ڣ��÷�Ӧ�Ļ�ѧ����ʽ��____________��
(4)ʹ����84������Һ(��![]() )ʱ����һ��������ˮ��ϣ����ڿ����н���һ��ʱ�䣬ʹ
)ʱ����һ��������ˮ��ϣ����ڿ����н���һ��ʱ�䣬ʹ![]() ��
��![]() �������е�
�������е�![]() ��� ��Ӧ��Ŀ���ǵõ�ɱ������Ч�����õ�__________(����������)�����÷�Ӧ�����ӷ���ʽ����������_____________________________
��� ��Ӧ��Ŀ���ǵõ�ɱ������Ч�����õ�__________(����������)�����÷�Ӧ�����ӷ���ʽ����������_____________________________![]() ��
��
���𰸡��� �٢� �ڢ� ��ɫ���� ��ɫ 3Fe��4H2O(g)![]() Fe3O4��4H2 ������ ClO-+CO2+H2O��HClO+HCO3-
Fe3O4��4H2 ������ ClO-+CO2+H2O��HClO+HCO3-
��������
��1����ˮ��Һ���м��Ե������Ǽ��ˮ���Լ��Ե��Σ����������ӻ�笠����Ӻ����������ɵĻ�����Ϊ�Σ��������ʵ����ʷ����ж��ܷ�����Ӧ��
��2��ʳ���к���Ԫ�أ���Ԫ�ص���ɫ��ӦΪ��ɫ���棻
��3������ˮ������Ӧ����������������������
��4��̼�����Դ��ڴ����ᣬ������̼ͨ�����������Һ�з�Ӧ���ɴ������̼�����ƣ��ݴ��жϡ�
��1�������еĵ�ζƷ�����Σ���Ҫ�ɷ��Ȼ��ƣ����ڼ��棨��Ҫ�ɷ�̼���ƣ����۰״ף���Ҫ�ɷ����ᣩ�����������ʷֱ�����ˮ����ˮ��Һ���м��Ե��Ǣڼ��棨��Ҫ�ɷ�̼���ƣ���̼�������ˮ����Һ�Լ��ԣ���Ҫ�ɷ�������������Ǣ��Σ���Ҫ�ɷ��Ȼ��ƣ����ڼ��棨��Ҫ�ɷ�̼���ƣ������Ӧ��������̼���ƺ����ᷴӦ���ɴ����ơ�������̼��ˮ���ʴ�Ϊ���ڣ��٢ڣ��ڢۣ�
��2��ʳ�β�����������Ȼ���Ļ����ϣ��۲쵽�������ǻ�ɫ���棬�ñ仯��Ϊ��ɫ��Ӧ��
��3��մ��ˮ�����������ڸ��»����ϻᷢ�ڣ��÷�Ӧ�Ļ�ѧ����ʽ��3Fe��4H2O(g)![]() Fe3O4��4H2��
Fe3O4��4H2��
��4������̼�������ǿ�ڴ����ᣬʹ�á�84������Һ����NaClO��ʱ����һ��������ˮ��ϣ����ڿ����н���һ��ʱ�䣬ʹNaClO��H2O�������е�CO2��ַ�Ӧ��Ŀ���ǵõ�ɱ������Ч�����õ��Ǵ����ᣬ��Ӧ�����ӷ���ʽΪClO-+CO2+H2O��HClO+HCO3-��
�ʴ�Ϊ�������HClO��HCO3-��


 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܼ��������������Ч��ʩ�� (�� ��
������ú̿��ȼ�� �ڰѹ������̴����� �ۻ�ʯȼ������ �����Ѿ��ữ�������м�ʯ�� ����������Դ
A. �٢ڢۢ� B. �٢ۢ� C. �ڢۢ� D. �ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ0.1 mol��L��1 NaOH��Һ470 mL��0.5 mol��L��1������Һ500 mL��������������Һ����������ش��������⣺

(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����____(�����)������������Һ�����õ��IJ���������____ (����������)��
(2)���в����У�����ƿ�����߱��Ĺ�����___________(�����)��
A������һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E����ȡһ�������Һ��
F�����������ܽ��������
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��______0.1 mol��L��1(����ڡ������ڡ���С�ڡ�)��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��Ũ��������Ϊ________mL(����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����עӪ��ƽ��������ʹ��ҩ�������������Ľ��������������������ʣ�
A��ά����C B����֬ C����ù�� D�������
�����������ѡ��ǡ����ѡ������ĸ������ա�
�������������ܺʹ������õ�������______________________��
��ΪԤ����״���״�������ʳ���м����������______________________��
������ֹ����ϸ����������Ҫ��������__________________________��
���������߲ˡ�ˮ���������л�ԭ�Ե�������______________________��
��2�����Ź�ҵ�ķ�չ�����Ľ��������������ע������Ⱦ���⡣
������������ѭ����������������Դ����ԭ�������������ռ���ʵ������ԭ��Ĵ�ʩ֮һ��������ͼ��ʾ��־���������ռ�����________(����ĸ����)��

A���ɻ����� B���к����� C����������
���ҹ����з����������������ձ��������������ʲ�������Ҫ��Ⱦ�����______(����ĸ����)��
A���������� B��������̼ C���������� D�����������
��ʹ��Pb2�����������ˮ��������Σ�����彡����ijұ����������Pb2����������ķ�ˮ�������˷�ˮ�ķ����Ǽ�������Na2S��ʹPb2����������ȥ����д���÷�Ӧ�����ӷ���ʽ��______________________________________________��
��3����������������ͷ�չ�����ʻ���������ʹ�ò��Ͽ��Խ�Լ��Դ��
�����������еij������������ںϽ����________(����ĸ����)��
A��������̥ B������� C��ˮ��
���ҹ�����Ա���ĺ������Ҫ���ɾ����ܶ�С��ǿ�ȸߡ���ʴ���������������ܵ���������Ƴɵ�����Щ��������__________(����ĸ����)��
A��þ���Ͻ� B���ϳ���ά C����Ȼ��ά
��4��ijͬѧȡһ�Ź�������������ͼװ����������ʴʵ�顣�����۲쵽�Թ�������������Һ���������˹������������ķ�ӦʽΪFe��2e��===Fe2�����������ķ�ӦʽΪ__________________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O��ij�о���ѧϰС������ͼװ�ý��������ᾧ�����ȷֽ�IJ��ֲ������֤����ʵ�顣��ش��������⡣

�����ϲ��ġ�
�����ᾧ����101 ��ʱ��ʼ�ۻ���150 ��ʱ��ʼ������175 ��ʱ��ʼ�ֽ⣻
������ƺͲ�����ƾ�Ϊ��ɫ�����
��1��������ͼ��ʾ��װ����ͨ��ʵ�������ᾧ��IJ��ַֽ������װ��B�пɹ۲쵽������ð���ҳ���ʯ��ˮ��������ɴ˼�ͬѧ�жϲ��ᾧ��ֽ�IJ�������CO2�����������ͬѧ�������䷴�Ե����ɿ�����______________________________________��
��2����ͬѧ��Ϊ���ᾧ��ֽ�IJ����к���CO��Ϊ������֤��XӦѡ��________(�ѧʽ)Ũ��Һ��װ��D��������____________________��
��3��ʵ��������漰���²������ٵ�ȼװ��A���ľƾ��ƣ���Ϩ��װ��A���ľƾ��ƣ��۵�ȼװ��E���ľƾ��ƣ���Ϩ��װ��E���ľƾ��ơ���4���������ȵ����˳��Ϊ____________(�����)����ȼE���ƾ���ǰ����Ҫ���еIJ�����______________��
��4��ʵ������з���װ��E�к�ɫ��ĩ���ɫ��װ��F���к�ɫ���������������װ��F�еĹ���Ϊ������������װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
��5����ͬѧ�õζ����ⶨ���ᾧ���нᾧˮ�ĺ��������������в�����
����һ���÷�����ƽ��ȡ3.15 g�����ĸò��ᾧ�������Ƴ�250 mL��Һ��
�����������Һ����ȡ25.00 mL���������Һ����ƿ�������������������ữ��
��������ȡ0.100 mol��L��1������KMnO4��Һ�����еζ������ν�����±���ʾ��
��һ�� | �ڶ��� | ������ | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪�ζ���Ӧ�����ӷ���ʽΪ��MnO![]() ��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
�����Ʋ�����Һ�IJ������������ǣ������������ձ�������ˮ�ܽ�������Һת����________��ϴ�ӣ����ݣ�ҡ�ȡ�
��ͨ������ȷ��x��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ����������������ء�����˵����ȷ����
A.���ࡢ�����ʶ�����Ȼ�л��߷��ӻ�����
B.���ķ���ϩ����ʴ.������������Ӧ�����ڱ�Ϳ��
C.SiO2���е�����.�������������ά����
D.����̿����ȥ����ζ��ɱ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y��Z��M��G��Q�����ֶ���������Ԫ�أ�ԭ��������������X��Z��Q�ĵ����ڳ����³���̬��Y��ԭ������������������Ӳ�����2����X��Mͬ���壬Z����̬�⻯������������������ˮ���ﷴӦ��G�ǵؿ��к�����ߵĽ���Ԫ�ء���ش��������⣺
(1)Q��Ԫ�ط���Ϊ______��Y��Z��M��G����Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����(дԪ�ط���)_______��
(2)Y��Ԫ�����ڱ��е�λ��Ϊ_______________��Y����Ԫ���γɵĶ�Ԫ������ĵ���ʽΪ_________________________��
(3)����Ԫ�ص�����������Ӧ��ˮ����������ǿ����(д��ѧʽ)_____________��
(4)����Y��Ԫ��Z������������Ӧˮ�����Ũ��Һ������Ӧ�Ļ�ѧ����ʽΪ_________��
(5)Z��G��ɵĻ�����GZ�������������������Ԫ������ҵ����G�������Y���ʺ�Z�����ڸ������Ʊ�GZ������G���������Y���ʵ����ʵ���֮��Ϊ1��3����÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ú��������Ȼ��Ƶ��Ȼ��ƹ��壬����100 mL 1mol��L��1���Ȼ�����Һ�����������IJ������������ݷ�����������ش��������⣺
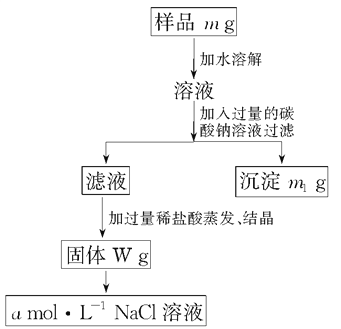
��1������������Ʒm g��������������Ϊ��_____________��
��2������ʱ���������������ǣ�__________________��
��3����������ʱӦ��Һ�����__________�м��ȣ��ȼ�����________ʱ��ֹͣ���ȡ�
��4������Ʒ����Һ�м��������Na2CO3��Һ��������__________________����Ӧ�Ļ�ѧ����ʽ��______________________________________��
��5������Һ�м��������������____________________________________��
��Ӧ�����ӳ�ʽ��_______________________________________��
��6������100 mL 1 mol��L��1��NaCl��Һʱ��Ӧ��W g�����г�ȡNaCl������Ϊ________������ʱӦ��________�н��ж�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com