
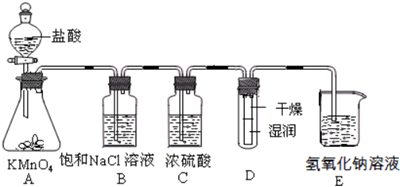
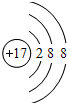
 ��
������ ��1�������Ӻ�����17�����ӣ�������3�����Ӳ㣬����ֱ����ɵ���2��8��8���ݴ�д�������ӽṹʾ��ͼ��
��2��Ũ������лӷ��ԣ������ܹ��ٽ�Ũ����Ļӷ���������ȡ�������к����Ȼ����ˮ�������ñ���ʳ��ˮ��ȥ�Ȼ��⣬��Ũ��������ˮ��
��3���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��4��������������ʹ��ɫ������ɫ��HClOʹ��ɫ��������ɫ��ˮ���ӵ��˶���������
��� �⣺��1�������Ӻ�����17�����ӣ�������3�����Ӳ㣬����ֱ����ɵ���2��8��8���ṹʾ��ͼΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2��Ũ������лӷ��ԣ������ܹ��ٽ�Ũ����Ļӷ���������ȡ�������к����Ȼ����ˮ������Ҫ�õ����﴿����������Ӧ����ͨ��ʢ�б���ʳ��ˮ��ϴ��ƿ��ȥ�Ȼ��⣬Ȼ��ͨ��ʢ��Ũ�����ϴ��ƿ��ȥˮ��
�ʴ�Ϊ����ȥ�����е��Ȼ������壻����������
��3���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ����ѧ����ʽ��Cl2+2NaOH=NaCl+NaClO+H2O��
�ʴ�Ϊ��Cl2+2NaOH=NaCl+NaClO+H2O��
��4������������ʹ��ɫ������ɫ��HClOʹ��ɫ��������ɫ����һ��ʱ���ˮ�������˶�������ĺ�ɫֽ���У���ʵ�鲻������
�����ʵ�齫������ͨ��װ�и���ĺ�ɫֽ�����Թܣ���ͨ��װ��ʪ��ĺ�ɫֽ�����Թ��У�
�ʴ�Ϊ����������ʵ�������Cl2��ʪ���ɫֽ���е�ˮ��Ӧ����HClOʹʪ��ĺ�ɫֽ����ɫ�����ﲿ��û��HClO������ֽ������ɫ������һ��ʱ�䣬����ˮ�����˶��ᵼ������ֽ��ʪ�����ɫ��
��������ͨ��װ�и���ĺ�ɫֽ�����Թܣ���ͨ��װ��ʪ��ĺ�ɫֽ�����Թ��У�
���� ���⿼����������ȡʵ��װ�ü����������ʣ���ȷװ�õ����ü�װ���з����Ļ�ѧ��Ӧ���ɽ�𣬲��ؿ���ѧ������ʵ�����������Ŀ�ѶȲ���


 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� ��� | �¶ȣ��棩 | ��ʼ���ʵ�����mol�� | ƽ�����ʵ�����mol�� | |
| CH3OH��g�� | CH3OCH3��g�� | H2O��g�� | ||
| �� | 387 | 0.20 | 0.080 | 0.080 |
| �� | 387 | 0.40 | a | b |
| �� | 207 | 0.20 | 0.090 | 0.090 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�dzʵ����Եģ��������ǿ��Դ���� | |
| B�� | ���ˮ�м���FeCl3������Һ�������������Һ�ʺ��ɫʱ���õ�Fe��OH��3���� | |
| C�� | �峿����������Ҷ��ķ�϶���Բ��������ЧӦ��˵��������һ�ֽ��� | |
| D�� | ������������Һ�ͽ���ķ��������ö����ЧӦ�����ڻ�ѧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״����6.72L NH3������ԼΪ5.1g | |
| B�� | 10g D216O����������������Ϊ4NA | |
| C�� | 2.3g������ȫ�����������ʧȥ�ĵ�����ĿΪ0.2NA | |
| D�� | 150mL1mol/L�Ȼ�����Һ��50mL1mol/L AlCl3��Һ�������ӵ����ʵ���Ũ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȼ�����Һ�м��������ˮ��Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | |
| B�� | ̼������ڴ���CaCO3+2H+�TCa2++CO2��+H2O | |
| C�� | �Ȼ�������Һ��ͨ��������Fe2++Cl2�TFe3++2Cl- | |
| D�� | �����ʯ��ˮ��ϡ���ᷴӦCa��OH��2+2H+�TCa2++2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaClˮ��Һ | B�� | ���� | C�� | �� | D�� | KOH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧ�� | H-H | C-O | C O | H-O | C-H |
| E/��kJ��mol-1�� | 436 | 343 | 1076 | 465 | 413 |
| A�� | 99��-41 | B�� | +99��+41 | C�� | -99��+41 | D�� | -99��41 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com