
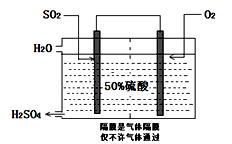
I£®ÄæĒ°£¬ĪŅ¹ś²ÉÓĆ”°½Ó“„·Ø”±ÖĘĮņĖį£¬Éč±øČēĶ¼ĖłŹ¾£ŗ

£Ø1£©Ķ¼ÖŠÉč±øAµÄĆū³ĘŹĒ_____________ øĆÉč±øÖŠÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓŠ¹Ų½Ó“„·ØÖĘĮņĖįµÄĻĀĮŠĖµ·ØÖŠ£¬²»ÕżČ·µÄŹĒ______________”£
A£®¶žŃõ»ÆĮņµÄ½Ó“„Ńõ»ÆŌŚ½Ó“„ŹŅÖŠ·¢Éś
B£®ĪüŹÕĖžÓĆÅضČĪŖ98.3%ÅØĮņĖįĪüŹÕČżŃõ»ÆĮņ
C£®ģŃÉÕŗ¬Įņ48%µÄ»ĘĢśæóŹ±£¬ČōFeS2ĖšŹ§ĮĖ2%£¬ŌņSĖšŹ§2%
D£®B×°ÖĆÖŠ·“Ó¦µÄĢõ¼žÖ®Ņ»ĪŖ½ĻøßĪĀ¶ČŹĒĪŖĮĖĢįøßSO2µÄ×Ŗ»ÆĀŹ
E£®ĮņĖį¹¤ŅµÖŠŌŚ½Ó“„ŹŅ°²×°ČČ½»»»Ę÷ŹĒĪŖĮĖĄūÓĆSO3×Ŗ»ÆĪŖH2SO4Ź±·Å³öµÄČČĮæ
£Ø3£©æĘŃŠ¹¤×÷ÕßæŖ·¢ĮĖÖʱøSO2£¬ŌŁÓƵē»ÆѧŌĄķÉś²śĮņĖįµÄ·½·Ø£¬×°ÖĆČēĶ¼£ŗĪŖĮĖĪČ¶Ø¼¼ŹõÉś²ś£¬ĮņĖįµÄÅضČÓ¦Ī¬³Ö²»±ä£¬ŌņĶØČėµÄSO2ŗĶĖ®µÄÖŹĮæ±ČĪŖ ”£
II. “æ¼īŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ÄæĒ°ÖĘ¼ī¹¤ŅµÖ÷ŅŖÓŠ ”°°±¼ī·Ø”±ŗĶ”°ĮŖŗĻÖĘ¼ī·Ø”±Į½ÖÖ¹¤ŅÕ”£Ēė°“ŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø1£©”°°±¼ī·Ø”±²śÉś“óĮæCaCl2·ĻĘśĪļ£¬ĒėŠ“³öøĆ¹¤ŅÕÖŠ²śÉśCaCl2µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø2£©Š“³ö”°ĮŖŗĻÖĘ¼ī·Ø”±ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
ӣ
£Ø3£©CO2ŹĒÖĘ¼ī¹¤ŅµµÄÖŲŅŖŌĮĻ£¬”°ĮŖŗĻÖĘ¼ī·Ø”±ÖŠCO2µÄĄ“Ō“ÓŚ £¬
”°°±¼ī·Ø”±ÖŠCO2Ą“Ō“ÓŚ £»
¢ń£Ø1£©·ŠĢŚĀÆ £Ø1·Ö£©£¬4FeS2 +11O2 ![]() 2Fe2O3 + 8SO2 £Ø2·Ö£©
2Fe2O3 + 8SO2 £Ø2·Ö£©
£Ø2£©D E£Ø2·Ö”£ĘĄ·Ö±ź×¼Ķ¬Ē°£© £Ø3£©16©s29 £Ø2·Ö£©
¢ņ£Ø1£©2NH4Cl + Ca(OH)2 ![]() CaCl2 + 2NH3”ü+ 2H2O £Ø2·Ö£©
CaCl2 + 2NH3”ü+ 2H2O £Ø2·Ö£©
£Ø2£©NH3 + H2O + CO2 + NaCl£Ø±„ŗĶ£© £½ NaHCO3 ”ż+ NH4Cl£Ø2·Ö£©
2NaHCO3 ![]() Na2CO3 + CO2 ”ü+ H2O £Ø2·Ö)
Na2CO3 + CO2 ”ü+ H2O £Ø2·Ö)
£Ø3£©ŗĻ³É°±³§£Ø1·Ö£© ”£ģŃÉÕŹÆ»ŅŹÆ£Ø1·Ö£©
ĀŌ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
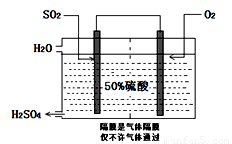
I£®ÄæĒ°£¬ĪŅ¹ś²ÉÓĆ”°½Ó“„·Ø”±ÖĘĮņĖį£¬Éč±øČēĶ¼ĖłŹ¾£ŗ
£Ø1£©Ķ¼ÖŠÉč±øAµÄĆū³ĘŹĒ_____________ øĆÉč±øÖŠÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓŠ¹Ų½Ó“„·ØÖĘĮņĖįµÄĻĀĮŠĖµ·ØÖŠ£¬²»ÕżČ·µÄŹĒ______________”£
A£®¶žŃõ»ÆĮņµÄ½Ó“„Ńõ»ÆŌŚ½Ó“„ŹŅÖŠ·¢Éś
B£®ĪüŹÕĖžÓĆÅضČĪŖ98.3%ÅØĮņĖįĪüŹÕČżŃõ»ÆĮņ
C£®ģŃÉÕŗ¬Įņ48%µÄ»ĘĢśæóŹ±£¬ČōFeS2ĖšŹ§ĮĖ2%£¬ŌņSĖšŹ§2%
D£®B×°ÖĆÖŠ·“Ó¦µÄĢõ¼žÖ®Ņ»ĪŖ½ĻøßĪĀ¶ČŹĒĪŖĮĖĢįøßSO2µÄ×Ŗ»ÆĀŹ
E£®ĮņĖį¹¤ŅµÖŠŌŚ½Ó“„ŹŅ°²×°ČČ½»»»Ę÷ŹĒĪŖĮĖĄūÓĆSO3×Ŗ»ÆĪŖH2SO4Ź±·Å³öµÄČČĮæ
£Ø3£©æĘŃŠ¹¤×÷ÕßæŖ·¢ĮĖÖʱøSO2£¬ŌŁÓƵē»ÆѧŌĄķÉś²śĮņĖįµÄ·½·Ø£¬×°ÖĆČēĶ¼£ŗĪŖĮĖĪČ¶Ø¼¼ŹõÉś²ś£¬ĮņĖįµÄÅضČÓ¦Ī¬³Ö²»±ä£¬ŌņĶØČėµÄSO2ŗĶĖ®µÄÖŹĮæ±ČĪŖ ”£
II. “æ¼īŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ÄæĒ°ÖĘ¼ī¹¤ŅµÖ÷ŅŖÓŠ ”°°±¼ī·Ø”±ŗĶ”°ĮŖŗĻÖĘ¼ī·Ø”±Į½ÖÖ¹¤ŅÕ”£Ēė°“ŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø1£©”°°±¼ī·Ø”±²śÉś“óĮæCaCl2·ĻĘśĪļ£¬ĒėŠ“³öøĆ¹¤ŅÕÖŠ²śÉśCaCl2µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø2£©Š“³ö”°ĮŖŗĻÖĘ¼ī·Ø”±ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
ӣ
£Ø3£©CO2ŹĒÖĘ¼ī¹¤ŅµµÄÖŲŅŖŌĮĻ£¬”°ĮŖŗĻÖĘ¼ī·Ø”±ÖŠCO2µÄĄ“Ō“ÓŚ £¬
”°°±¼ī·Ø”±ÖŠCO2Ą“Ō“ÓŚ £»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģ¼ŖĮÖŹ”ĘÕĶØ֊ѧøßČżĻĀѧʌʌ֊½Ģѧ֏Įæ¼ģ²āĄķ×Ū»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
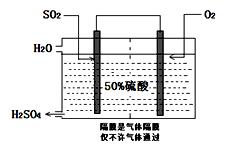
I£®ÄæĒ°£¬ĪŅ¹ś²ÉÓĆ”°½Ó“„·Ø”±ÖĘĮņĖį£¬Éč±øČēĶ¼ĖłŹ¾£ŗ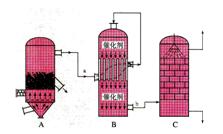
£Ø1£©Ķ¼ÖŠÉč±øAµÄĆū³ĘŹĒ_____________ øĆÉč±øÖŠÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓŠ¹Ų½Ó“„·ØÖĘĮņĖįµÄĻĀĮŠĖµ·ØÖŠ£¬²»ÕżČ·µÄŹĒ______________”£
A£®¶žŃõ»ÆĮņµÄ½Ó“„Ńõ»ÆŌŚ½Ó“„ŹŅÖŠ·¢Éś | B£®ĪüŹÕĖžÓĆÅضČĪŖ98.3%ÅØĮņĖįĪüŹÕČżŃõ»ÆĮņ |
| C£®ģŃÉÕŗ¬Įņ48%µÄ»ĘĢśæóŹ±£¬ČōFeS2ĖšŹ§ĮĖ2%£¬ŌņSĖšŹ§2% | |
| D£®B×°ÖĆÖŠ·“Ó¦µÄĢõ¼žÖ®Ņ»ĪŖ½ĻøßĪĀ¶ČŹĒĪŖĮĖĢįøßSO2µÄ×Ŗ»ÆĀŹ |
 °±¼ī·Ø”±ÖŠCO2Ą“Ō“ÓŚ £»
°±¼ī·Ø”±ÖŠCO2Ą“Ō“ÓŚ £»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗž±±Ź”°ĖŹŠøßȿȿŌĀĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
I£®ÄæĒ°£¬ĪŅ¹ś²ÉÓĆ”°½Ó“„·Ø”±ÖĘĮņĖį£¬Éč±øČēĶ¼ĖłŹ¾£ŗ

£Ø1£©Ķ¼ÖŠÉč±øAµÄĆū³ĘŹĒ_____________£¬øĆÉč±øÖŠÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ??????????? ”£
£Ø2£©ÓŠ¹Ų½Ó“„·ØÖĘĮņĖįµÄĻĀĮŠĖµ·ØÖŠ£¬²»ÕżČ·µÄŹĒ_____£ØĢī×ÖÄøŠņŗÅ£©”£
A£®¶žŃõ»ÆĮņµÄ½Ó“„Ńõ»ÆŌŚ½Ó“„ŹŅÖŠ·¢Éś
B£®ĪüŹÕĖžÓĆÅضČĪŖ98.3%ÅØĮņĖįĪüŹÕČżŃõ»ÆĮņ
C£®ģŃÉÕŗ¬Įņ48%µÄ»ĘĢśæóŹ±£¬ČōFeS2ĖšŹ§ĮĖ2%£¬ŌņSĖšŹ§2%
D£®B×°ÖĆÖŠ·“Ó¦µÄĢõ¼žÖ®Ņ»ĪŖ½ĻøßĪĀ¶ČŹĒĪŖĮĖĢįøßSO2µÄ×Ŗ»ÆĀŹ
E£®ĮņĖį¹¤ŅµÖŠŌŚ½Ó“„ŹŅ°²×°ČČ½»»»Ę÷ŹĒĪŖĮĖĄūÓĆSO3×Ŗ»ÆĪŖ H2SO4Ź±·Å³öµÄČČĮæ
II£®“æ¼īŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ÖĘ¼ī¹¤ŅµÖ÷ŅŖÓŠ”°°±¼ī·Ø”±(Ė÷¶ūĪ¬·Ø)ŗĶ”°ĮŖŗĻÖĘ¼ī·Ø”±(ŗīŹĻÖĘ¼ī·Ø)Į½ÖÖ¹¤ŅÕ”£Ēė°“ŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø3£©CO2ŹĒÖĘ¼ī¹¤ŅµµÄÖŲŅŖŌĮĻ£¬”°ĮŖŗĻÖĘ¼ī·Ø”±ÖŠCO2µÄĄ“Ō“ÓŚ £¬”°°±¼ī·Ø”±ÖŠCO2Ą“Ō“ÓŚ ”£
£Ø4£©°±¼ī·ØµÄŌ×ÓĄūÓĆĀŹ(Ō×ÓĄūÓĆĀŹ=ĘŚĶū²śĪļµÄ×ÜÖŹĮæÓėÉś³ÉĪļµÄ×ÜÖŹĮæÖ®±Č) ??????? ”£
£Ø5£©Š“³ö”°ĮŖŗĻÖĘ¼ī·Ø”±ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ?????????????????????????????? ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğ¼ŖĮÖŹ”øßČżĻĀѧʌʌ֊½Ģѧ֏Įæ¼ģ²āĄķ×Ū»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
I£®ÄæĒ°£¬ĪŅ¹ś²ÉÓĆ”°½Ó“„·Ø”±ÖĘĮņĖį£¬Éč±øČēĶ¼ĖłŹ¾£ŗ
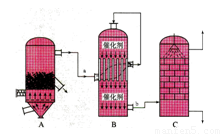
£Ø1£©Ķ¼ÖŠÉč±øAµÄĆū³ĘŹĒ_____________ øĆÉč±øÖŠÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓŠ¹Ų½Ó“„·ØÖĘĮņĖįµÄĻĀĮŠĖµ·ØÖŠ£¬²»ÕżČ·µÄŹĒ______________”£
A£®¶žŃõ»ÆĮņµÄ½Ó“„Ńõ»ÆŌŚ½Ó“„ŹŅÖŠ·¢Éś
B£®ĪüŹÕĖžÓĆÅضČĪŖ98.3%ÅØĮņĖįĪüŹÕČżŃõ»ÆĮņ
C£®ģŃÉÕŗ¬Įņ48%µÄ»ĘĢśæóŹ±£¬ČōFeS2ĖšŹ§ĮĖ2%£¬ŌņSĖšŹ§2%
D£®B×°ÖĆÖŠ·“Ó¦µÄĢõ¼žÖ®Ņ»ĪŖ½ĻøßĪĀ¶ČŹĒĪŖĮĖĢįøßSO2µÄ×Ŗ»ÆĀŹ
E£®ĮņĖį¹¤ŅµÖŠŌŚ½Ó“„ŹŅ°²×°ČČ½»»»Ę÷ŹĒĪŖĮĖĄūÓĆSO3×Ŗ»ÆĪŖH2SO4Ź±·Å³öµÄČČĮæ
£Ø3£©æĘŃŠ¹¤×÷ÕßæŖ·¢ĮĖÖʱøSO2£¬ŌŁÓƵē»ÆѧŌĄķÉś²śĮņĖįµÄ·½·Ø£¬×°ÖĆČēĶ¼£ŗĪŖĮĖĪČ¶Ø¼¼ŹõÉś²ś£¬ĮņĖįµÄÅضČÓ¦Ī¬³Ö²»±ä£¬ŌņĶØČėµÄSO2ŗĶĖ®µÄÖŹĮæ±ČĪŖ ”£
II. “æ¼īŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ÄæĒ°ÖĘ¼ī¹¤ŅµÖ÷ŅŖÓŠ ”°°±¼ī·Ø”±ŗĶ”°ĮŖŗĻÖĘ¼ī·Ø”±Į½ÖÖ¹¤ŅÕ”£Ēė°“ŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø1£©”°°±¼ī·Ø”±²śÉś“óĮæCaCl2·ĻĘśĪļ£¬ĒėŠ“³öøĆ¹¤ŅÕÖŠ²śÉśCaCl2µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø2£©Š“³ö”°ĮŖŗĻÖĘ¼ī·Ø”±ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
ӣ
£Ø3£©CO2ŹĒÖĘ¼ī¹¤ŅµµÄÖŲŅŖŌĮĻ£¬”°ĮŖŗĻÖĘ¼ī·Ø”±ÖŠCO2µÄĄ“Ō“ÓŚ £¬
”°°±¼ī·Ø”±ÖŠCO2Ą“Ō“ÓŚ £»
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com