

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����



| ||
| ||

| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
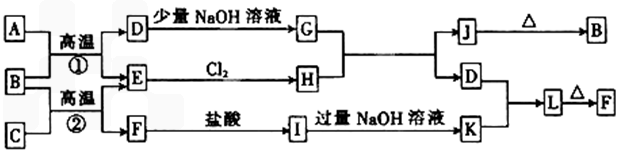
��10�֣���ͼ��һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ�������
����ȥ�����³�ѹ�£�AΪ��ɫ�ж����壬BΪ����ɫ��ĩ��C��EΪ�������ʣ���Ӧ�٢ھ�Ϊ��ҵ�ϵ���Ҫ��Ӧ��
��ش��������⣺
��1��D�� ��K�� �����ѧʽ��
��2��д��B��C���·�Ӧ����E��F�Ļ�ѧ����ʽ��
��
��3��д��D��J��Һ��Ӧ����G�����ӷ���ʽ��
��
��4��д��ͼ��I����L�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��Ƚ���ʡ�߶���һѧ����ĩģ�⿼�Ի�ѧ�����ޣ��Ծ� ���ͣ������
��6�֣���ͼ��һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʱ���ȥ����Ӧ�ٳ���Ӧ����Ұ�⺸�Ӹֹ죬AΪ�ճ������г����Ľ������ʣ� GΪ����ɫ���壬F�Ǻ�ˮ�к����ε���Ҫ�ɷ֣�JΪD��G��Ӧ�����γɵ�ˮ��Һ��

��ش��������⣺
��1��H�Ļ�ѧʽΪ_______________����2����Ӧ���ڹ�ҵ��ͨ����֮Ϊ__________��ҵ��
��3��д����Ӧ�۵����ӷ���ʽ ��
��4��д����Ӧ�ڵĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��10�֣���ͼ��һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ�������
����ȥ�����³�ѹ�£�AΪ��ɫ�ж����壬BΪ����ɫ��ĩ��C��EΪ�������ʣ���Ӧ�٢ھ�Ϊ��ҵ�ϵ���Ҫ��Ӧ��

��ش��������⣺
��1��D�� ��K�� �����ѧʽ��
��2��д��B��C���·�Ӧ����E��F�Ļ�ѧ����ʽ��
��
��3��д��D��J��Һ��Ӧ����G�����ӷ���ʽ��
��
��4��д��ͼ��I����L�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com