
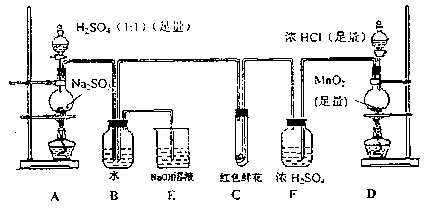
 Na2SO4��SO2����2H2O�����ò��������������ƾ��ơ�Բ����ƿ����Һ©��
Na2SO4��SO2����2H2O�����ò��������������ƾ��ơ�Բ����ƿ����Һ©��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

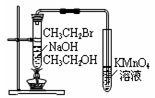
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
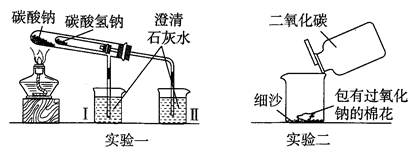
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
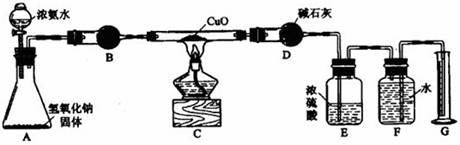
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ֱ�ӷ������Ǽ��Ͻ��м��� |
| B�������ڼ���ǰδ��ȫ�����ʹ�ⶨ�Ľ��ƫ�� |
| C�������¶ȹ��ߣ�ʹһ��������ͭ�ֽ⣬��ʹ�ⶨ���ƫ�� |
| D��ʵ�鲽���˳��Ϊ����������ĥ�����ȡ���ȴ���������ټ��ȡ���ȴ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ�Ϸ����Ŵ�С��ȵ�ֽƬ��Ȼ��NaOH�������ֽƬ�Ͻ��г��� |
| B���ѳƵõ�NaOH������������ˮ���ձ��У��ܽ⡢��ȴ���ٰ���Һ��������ƿ�� |
| C��������ˮϴ���ձ��Ͳ�����2~3�Σ�ϴ��ҺҲ��������ƿ�� |
| D����̶�1��2cmʱ���ý�ͷ�ιܼ�������ˮ��ֱ��Һ�尼����͵�ǡ����̶����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ~3��һ��ת�����С�
~3��һ��ת�����С�| ʵ����� | �ζ���Һ����ʼ���� | �ζ���Һ���յ���� |
| 1 | 1.32mL | 23.36mL |
| 2 | 2.26mL | 24.22mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȡ����ˮʱ��Ϊ��ֹ��ƿ�ڲ�����������Ӧ��������ƿ�м��뼸Ƭ���Ƭ |
| B���ⶨδ֪NaOH��ҺŨ��ʱ����ʽ�ζ������ñ���Һ��ϴ2��3�� |
| C������0.1mol/L��H2SO4��Һʱ������ȡ��ŨH2SO4��������ƿ�м�ˮϡ�� |
| D��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ������ʪ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ����ʱ����NaOH������������ڵ���ֽ�ϣ��Ƶ�����Ϊ10.2g |
| B����50mL��ʽ�ζ�����ȡKOH��Һ�����Ϊ36.60mL |
C����ʪ���pH��ֽ��ϡ ������Һ��pH��pH��3.52 ������Һ��pH��pH��3.52 |
| D����10mL��Ͳ������ȡNaCl��Һ�����Ϊ9.2mL |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com