
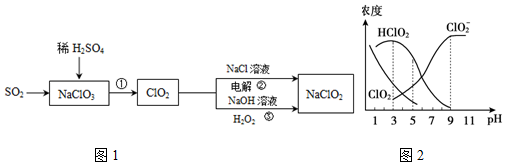
·ÖĪö £Ø1£©øł¾Ż»ÆŗĻĪļÖŠ»ÆŗĻ¼Ū“śŹżŗĶĪŖ0æÉµĆ£»ŌŖĖŲ“¦ÓŚÖŠ¼ä¼ŪĢ¬ÓŠŃõ»ÆŠŌŗĶ»¹ŌŠŌ£»
£Ø2£©·“Ó¦¢ŁÖŠĀČĖįÄĘÓÉÓŠŃõ»ÆŠŌ£¬¶žŃõ»ÆĮņÓŠ»¹ŌŠŌ£¬ŌŚĖįŠŌ»·¾³ĻĀÉś³ÉClO2ŗĶĮņĖįøłĄė×Ó£»øł¾Ż·“Ó¦ÖŠŌŖĖŲµĆŹ§µē×ÓŹżĻąµČæÉµĆ£»
£Ø3£©µē½ā”±ÖŠ£¬½«¶žŃõ»ÆĀČĘųĢåĶعżµĆµē×Ó»ńµĆŃĒĀČĖįøł£¬µĆµē×ÓŅ»¼«ĪŖŅõ¼«£»
£Ø4£©·“Ó¦¢Ū·¢Éś·“Ó¦ĪŖClO2ŗĶH2O2ŗĶĒāŃõ»ÆÄĘÉś³ÉŃĒĀČĖįÄĘŗĶŃõĘųµÄ·“Ó¦£»»¹Ō¼ĮĪŖ¹żŃõ»ÆĒā£¬Ńõ»Æ¼ĮĪŖClO2£»
£Ø5£©a”¢ÓÉĶ¼æÉŅŌµĆ³ö£ŗ¼īŠŌĢõ¼žĻĀClO2-ÅضČøߣ»
b”¢NaClO2ČÜŅŗ£¬ŅõĄė×ÓĖ®½ā£»
c”¢øł¾ŻĢāŅā£ŗHClO2ŗĶClO2¶¼¾ßÓŠĘÆ°××÷ÓĆ£¬½įŗĻĶ¼ÖŠHClO2ŗĶClO2µÄÅØ¶Č“óŠ”Ą“Č·¶ØŹ¹ÓĆøĆĘÆ°×¼ĮµÄ×ī¼ŃpH£»
d”¢pH£¾6Ź±£¬ÓÉĶ¼æÉÖŖ£¬ČÜŅŗÖŠÖ÷ŅŖŗ¬ClO2-£®
½ā“š ½ā£ŗ£Ø1£©»ÆŗĻĪļÖŠ»ÆŗĻ¼Ū“śŹżŗĶĪŖ0£¬NaClO2ÖŠÄĘŌŖĖŲ+1¼Ū£¬ŃõŌŖĖŲ-2¼Ū£¬ŌņĀČŌŖĖŲ+3¼Ū£»ŌņNaClO2¾ßÓŠŃõ»ÆŠŌ”¢»¹ŌŠŌ£»
¹Ź“š°øĪŖ£ŗ+3£»Ńõ»ÆŠŌ”¢»¹ŌŠŌ£»
£Ø2£©·“Ó¦¢ŁÖŠĄė×Ó·½³ĢŹ½ŹĒ£ŗ2ClO3-+SO2ØTSO42-+2ClO2£Ø»ņ2ClO3-+SO2+2H+ØT2HSO4-+2ClO2£©£»±ź×¼×“æöĻĀ£¬22.4 L ClO2ĘųĢ弓ĪŖ1mol£¬·“Ó¦ÖŠClŌŖĖŲ»ÆŗĻ¼ŪÓÉ+5¼Ū½µµĶµ½+4¼Ū£¬Ōņ×ŖŅĘ1molµē×Ó£¬ŌņĮņŌŖĖŲ»ÆŗĻ¼Ū“Ó+4¼ŪÉżøßµ½+6¼Ū£¬ŌņSO2ÓŠ0.5mol£¬ÖŹĮæĪŖ0.5mol”Į64g/mol=32g£»
¹Ź“š°øĪŖ£ŗ2ClO3-+SO2ØTSO42-+2ClO2£Ø»ņ2ClO3-+SO2+2H+ØT2HSO4-+2ClO2£©£»32g£»
£Ø3£©µē½ā”±ÖŠ£¬½«¶žŃõ»ÆĀČĘųĢåĶعżµĆµē×Ó»ńµĆŃĒĀČĖįøł£¬µĆµē×ÓŅ»¼«ĪŖŅõ¼«£»
¹Ź“š°øĪŖ£ŗŅõ¼«£»
£Ø4£©·“Ó¦¢Ū·¢Éś·“Ó¦ĪŖ2ClO2+H2O2+2NaOHØT2NaClO2+2H2O+O2£»ClO2ÖŠĀČŌŖĖŲ»ÆŗĻ¼Ū½µµĶµĆµē×Ó×÷Ńõ»Æ¼Į£¬H2O2ÖŠŃõŌŖĖŲ»ÆŗĻ¼ŪÉżøߏ§µē×Ó×÷»¹Ō¼Į£¬øĆ·“Ó¦ÖŠ»¹Ō¼ĮÓėŃõ»Æ¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ£ŗ1£ŗ2£»
¹Ź“š°øĪŖ£ŗ2ClO2+H2O2+2NaOHØT2NaClO2+2H2O+O2£»1£ŗ2£»
£Ø5£©a”¢ÓÉĶ¼æÉŅŌµĆ³ö£ŗ¼īŠŌĢõ¼žĻĀClO2-ÅضČøߣ¬¼“ŌŚ¼īŠŌĢõ¼žĻĀŃĒĀČĖįÄĘ½ĻĪČ¶Ø£¬¹ŹaÕżČ·£»
b”¢NaClO2ČÜŅŗ£¬ŅõĄė×ÓĖ®½ā£¬Ōņ$\frac{c£ØN{a}^{+}£©}{c£ØCl{{O}_{2}}^{-}£©}$£¾1£¬¹Źb“ķĪó£»
c”¢HClO2ŗĶClO2¶¼¾ßÓŠĘÆ°××÷ÓĆ£¬½įŗĻĶ¼ÖŠHClO2ŗĶClO2µÄÅضČŌ½“ó¼“ĪŖŹ¹ÓĆøĆĘÆ°×¼ĮµÄ×ī¼ŃpH£¬Ó¦øĆŹĒ4-5£¬¹Źc“ķĪó£»
d”¢pH£¾6Ź±£¬ÓÉĶ¼æÉÖŖ£¬ČÜŅŗÖŠÖ÷ŅŖŗ¬ClO2-£¬ŌņĶłNaClO2ČÜŅŗÖŠµĪČėĻ”ŃĪĖį£¬ČÜŅŗÖŠÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ£ŗClO2-+H+ØTHClO2£¬¹ŹdÕżČ·£»
¹Ź“š°øĪŖ£ŗa d£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄÖʱø£¬ĪŖøßĘµæ¼µć£¬Éę¼°Ń§ÉśµÄ·ÖĪöÄÜĮ¦”¢¼ĘĖćÄÜĮ¦µÄ漲飬°ŃĪÕŃõ»Æ»¹Ō·“Ó¦ŹĒ¹Ų¼ü£¬Ķ¬Ź±×¢Ņā¼°ŃĪĄąĖ®½āµČ£¬ĢāÄæÄѶČÖŠµČ£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1 mol?L-1 A1C13ČÜŅŗÖŠŗ¬ÓŠµÄAl3+ŹżÄæŠ”ÓŚNA | |
| B£® | ±ź×¼×“æöĻĀ£¬11 g3H216OÖŠŗ¬ÓŠµÄÖŹ×ÓŹżÄæĪŖ6NA | |
| C£® | 1 mol Li2OŗĶNa2O2µÄ»ģŗĻĪļÖŠŗ¬ÓŠµÄĄė×Ó×ÜŹż“óÓŚ3NA | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ£¬4.6 g NO2Ėłŗ¬µÄµŖŌ×ÓŹżÄæĪŖ0.1NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
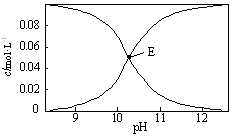 20”ꏱ£¬ÅäÖĘŅ»×éc£ØNa2CO3£©+c£ØNaHCO3£©=0.100mol•L-1µÄ»ģŗĻČÜŅŗ£¬ČÜŅŗÖŠc£ØHCO3-£©”¢c£ØCO32-£©ÓėpHµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ĻĀĮŠÖø¶ØČÜŅŗÖŠĪ¢Į£µÄĪļÖŹµÄĮæÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ£Ø””””£©
20”ꏱ£¬ÅäÖĘŅ»×éc£ØNa2CO3£©+c£ØNaHCO3£©=0.100mol•L-1µÄ»ģŗĻČÜŅŗ£¬ČÜŅŗÖŠc£ØHCO3-£©”¢c£ØCO32-£©ÓėpHµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ĻĀĮŠÖø¶ØČÜŅŗÖŠĪ¢Į£µÄĪļÖŹµÄĮæÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ£Ø””””£©| A£® | pH=9µÄČÜŅŗÖŠ£ŗc£ØHCO3-£©£¾c£ØH2CO3£©£¾c£ØCO32-£© | |
| B£® | c£ØHCO3-£©=c£ØCO32-£©µÄEµćČÜŅŗÖŠ£ŗc£ØOH-£©+c£ØCO32-£©£¾c£ØH+£©+c£ØH2CO3£©+0.050 mol•L-1 | |
| C£® | pH=11µÄČÜŅŗÖŠ£ŗc£ØNa+£©£¼2c£ØCO32-£©+c£ØHCO3-£© | |
| D£® | 0.100 mol•L-1µÄNa2CO3ČÜŅŗÖŠ£ŗc£ØH+£©+c£ØH2CO3£©+c£ØHCO3-£©=c£ØOH-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µČĢå»ż”¢µČĪļÖŹµÄĮæÅØ¶ČµÄNaXŗĶČõĖįHX»ģŗĻŗóµÄČÜŅŗpH£¾7£¬ŌņŅ»¶ØÓŠ£ŗc£ØNa+£©£¾c£ØHX£©£¾c£ØX-£©£¾c£ØH+£©£¾c£ØOH-£© | |
| B£® | 1L0.1mol•L-1CuSO4•£ØNH4£©2SO4•6H2OµÄČÜŅŗÖŠ£ŗc£ØSO42-£©£¾c£ØNH4+£©£¾c£ØCu2+£©£¾c£ØH+£©£¾c£ØOH-£© | |
| C£® | 0.1mol•L-1NaHCO3ČÜŅŗÖŠ£ŗc£ØNa+£©+c£ØH+£©+c£ØH2CO3£©”Łc£ØHCO3-£©+c£ØCO32-£©+c£ØOH-£© | |
| D£® | ĪļÖŹµÄĮæÅØ¶Č·Ö±šĪŖc1ŗĶc2µÄĮ½ÖÖ“×ĖįČÜŅŗ£¬ČōĘäpH·Ö±šĪŖaŗĶa+1£¬Ōņc1£¾10c2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 2NH3£¬øĆ·“Ó¦ÖŠµÄŃõ»Æ¼ĮŹĒN2£®
2NH3£¬øĆ·“Ó¦ÖŠµÄŃõ»Æ¼ĮŹĒN2£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
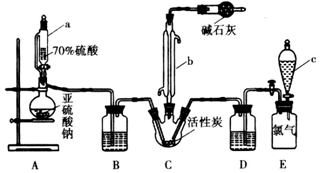
| ŹµŃé²½Öč | ŹµŃéĻÖĻó |
| a£®Č”ÉŁĮæøĆČÜŅŗ£¬¼Ó¼øµĪ¼×»ł³Č | ČÜŅŗ±äŗģÉ« |
| b£®Č”ÉŁĮæøĆČÜŅŗ¼ÓČČÅØĖõ£¬¼ÓCuʬŗĶÅØH2SO4£¬¼ÓČČ | ÓŠĪŽÉ«ĘųĢå²śÉś£¬ĘųĢåÓöæÕĘųæÉŅŌ±ä³Éŗģ×ŲÉ« |
| c£®Č”ÉŁĮæøĆČÜŅŗ£¬¼ÓBaCl2ČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É |
| d£®Č”ÉŁĮæøĆČÜŅŗ£¬¼ÓAgNO3ČÜŅŗ | ÓŠĪČ¶ØµÄ°×É«³ĮµķÉś³É£¬ĒŅ²»ČÜÓŚHNO3 |
| e£®Č”ÉŁĮæøĆČÜŅŗ£¬¼ÓNaOHČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É£¬µ±NaOH¹żĮæŹ±³Įµķ²æ·ÖČܽā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | c£ØOH-£©£¼c£ØH+£©£¼c£ØNH4+£©£¼c£ØSO42-£© | B£® | c£ØOH-£©£¼c£ØNH4+£©£¼c£ØSO42-£©£¼c£ØH+£© | ||
| C£® | c£ØSO42-£©+c£ØOH-£©£¾c£ØNH4+£©+c£ØH+£© | D£® | c£ØSO42-£©+c£ØOH-£©=c£ØNH4+£©+c£ØH+£© |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com