
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۹����Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��___________________________________��
��2��װ��B�б���ʳ��ˮ��������_______��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����______________________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���__________��
| | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
 ��Һ���жϸ���NaHSO
��Һ���жϸ���NaHSO ��Һ�Ƿ����__________����ǡ���������𡰷������ӷ���ʽ����ԭ��____________________�����ǡ����ý��ͣ���
��Һ�Ƿ����__________����ǡ���������𡰷������ӷ���ʽ����ԭ��____________________�����ǡ����ý��ͣ��� ��1��Ca(ClO) +4HCl(Ũ)=CaCl
+4HCl(Ũ)=CaCl +2Cl
+2Cl ��+2H
��+2H O��
O��
��2����ȥCl �е�HCl��B�г���©����Һ���������γ�ˮ����
�е�HCl��B�г���©����Һ���������γ�ˮ����
��3��d�� ��4���ƣ� ��5��E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ��
��6������δ��Ӧ����������HSO3-+Cl2+H2O=SO42-+2Cl-+3H+ HSO3-+H+=SO2��+H2O
���������������1��Ư�۾�����Ҫ�ɷ���Ca(ClO)2����ǿ�����ԣ���Ũ���ᷴӦ�ķ���ʽΪCa(ClO)2 + 4HCl(Ũ)= CaCl2 + 2Cl2��+ 2H2O��
��2�����ɵ������л����Ȼ������ͨ������ʳ��ˮ��ȥ����C�ж�������B��ѹǿ������ˮ����ѹ���Ӷ��γ�һ��ˮ����
��3��Ҫ����������Ư���ԣ�����������ɫ��������Ϊ��ȡ����������ˮ�����������Ҫ����������ѡ�ù轺����ˮ�Ȼ��ƣ�������ѡ���ʯ�Һ�Ũ���ᡣIIIһ���Ǹ������ɫ������������ȷ��ѡ����d��
��4����������ˮ���Ի�ɫ��
��5�������л��ܼ������Ϻ�ɫ����Ϊ�����ܶ�С��ˮ�ģ����ϲ����Ϻ�ɫ��
��6�������ж�����Ⱦ����������Fװ�õ����������ն����������HSO3�����л�ԭ�ԣ��ɻ�ԭ����������ʽΪHSO3�� + Cl2 + H2O �� SO42�� + 2Cl�� + 3H+����Ϊ��Ӧ����Һ�����ԣ����Ծ��п������ɶ���������Ⱦ���������Բ���ѡ�á�
���㣺��������ʵ��
�����������ۺ��Խ�ǿ����Ҫ����ѧ���Ļ���֪ʶ���ѶȲ���


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| a | b | c | d | |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡ��ˮ��ѧ������ѧ�ڶ������������Ծ����������� ���ͣ�ʵ����
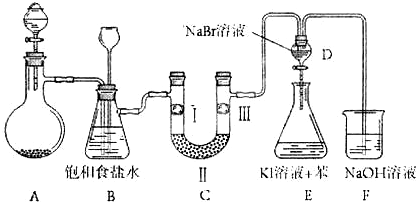
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���
���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��
__________________________________________________________________��
��װ��B�б���ʳ��ˮ��������_______________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����______________________________��
��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η���_______��
| | a | b | c | d |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и���1���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᡣ
��1�������й�ʵ�������ʵ����ʵ����������ȷ���� (�����)��
A��ʵ������Ũ����Ӧ��������ɫϸ��ƿ�У���������ͼ��ʾ��ǩ

B����50mL��Ͳ��ȡ5��6mLŨ����
C���к͵ζ�ʵ��ʱ����ƿϴ�Ӹɾ����ñ�Һ��ϴ����ע�����Һ
D�������Ȼ�̼��ȡ��ˮ�еĵ⣬��Һʱ�л���ӷ�Һ©�����¶˷ų�
E���ù㷺pH��ֽ���ij��Һ��pHΪ4��8
��2����ͼ��ʵ�����Ʊ�������̽�������Ƿ����Ư���Ե�ʵ��װ��(�гּ�����������ʡ��)��

��Aװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
��Bװ��������a�������� ��
��Bװ�õ������dz�ȥ�����л��е�HCl������ȫƿ�����ã�������a��Һ�治
������ʱ��˵�� ����ʱӦֹͣʵ�顣
��ʵ���й۲쵽 ��˵������������Ư���ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�����и���10���¿���ѧ�Ծ��������棩 ���ͣ�������
��10�֣���ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���

��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��
��
��2��װ��B�б���ʳ��ˮ�������� ��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ��
|
|
a |
b |
c |
d |
|
I |
�������ɫ���� |
�������ɫ���� |
ʪ�����ɫ���� |
ʪ�����ɫ���� |
|
II |
��ʯ�� |
�轺 |
Ũ���� |
��ˮ�Ȼ��� |
|
III |
ʪ�����ɫ���� |
ʪ�����ɫ���� |
�������ɫ���� |
�������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ���غ�ɫ��˵���ȵķǽ����Դ����塣��������װ��D��������Һ����װ��E�У����۲쵽�������� ��������
����ܡ����ܡ���˵����ķǽ�����ǿ�ڵ⣬ԭ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com