
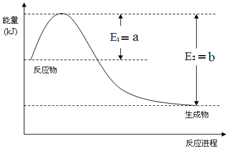
 ���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ����
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ�������� ��1������ͼ���N2��H2��Ӧ����1molNH3�ķ�Ӧ�ȣ��ٸ����Ȼ�ѧ��Ӧ����ʽ����д���
�ڸ��ݵ���غ㣬�ж�����Ũ�ȴ�С����ˮ�ĵ���ƽ�ⳣ��Ϊ��������ӵ�Ũ�Ȼ�������Ũ�ȵı�ֵ���Ȼ����Һ��笠�����ˮ�������ԣ�ˮ�������������Ũ��Ϊ10-5mol/L��pH=5��������Һ��ˮ�������������Ũ������ˮ�����ӻ�����õ���
��2������������Һ�м���NaI��Һ������AgI�������ټ�����AgI����ת��ΪAg2S��
��� �⣺��1������ͼ��֪��N2��H2��Ӧ����1molNH3�ų�������Ϊ��b-a��kJ���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-2��b-a��kJ•mol-1��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-2��b-a��kJ•mol-1��
�ڽ�n mol•L-1�İ�ˮ��m mol•L-1������������ϣ���Ӧ�����Һ�����ԣ���ˮ������������������Һ�ĵ���غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-������Һ�����ԣ���c��H+��=c��OH-��=10-7mol/L������Һ������Ũ�ȹ�ϵ��c��NH4+��=c��Cl-����c��H+��=c��OH-����c��NH4+��=c��Cl-��=$\frac{m}{2}$mol/L����Һ�а�ˮ�����������İ�ˮ��Ũ��c��NH3•H2O��=$\frac{n-m}{2}$mol/L����ˮ�еĵ��볣��Ϊ$\frac{c��N{{H}_{4}}^{+}��•c��{H}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$=$\frac{\frac{m}{2}��1{0}^{-7}}{\frac{n-m}{2}}$=$\frac{n��1{0}^{-7}}{n-m}$�����³�ѹ��pH������5��NH4Cl��HCl��Һ���Ȼ����Һ��笠�����ˮ�������Դٽ�ˮ�ĵ��룬��ˮ�������������Ũ�Ⱦ���10-5mol/L��������Һ�������Ӷ�ˮ�ĵ������������ã���HCl��Һ��ˮ�������������Ũ��Ϊ$\frac{1{0}^{-14}}{1{0}^{-5}}$=10-9mol/L������pH=5�������pH=5���Ȼ����Һ�У���ˮ���������C��H+��֮��10-9��10-5=10-4��1��
�ʴ�Ϊ��c��NH4+��=c��Cl-����c��H+��=c��OH-����$\frac{n��1{0}^{-7}}{n-m}$��10-4��1��
��2��25��C�£���0.1mol•L-1��NaI��Һ����μ���������0.1mol•L-1��������Һ��������������ӽ������AgI��ɫ��������AgI��Һ�м���Na2S��Һ���⻯��ת��ΪAg2S����ɫ����ת��Ϊ��ɫ��������Ӧ�����ӷ���ʽΪ��2AgI��s��+S2-��aq��=Ag2S��s��+2I-��aq����
�ʴ�Ϊ��������ɫ��������ɫ����ת��Ϊ��ɫ������2AgI��s��+S2-��aq��=Ag2S��s��+2I-��aq����
���� ���⿼�����Ȼ�ѧ����ʽ����ͼ������������ʵĵ���͵��볣���ļ��㡢������ת���ȣ���Ŀ�Ѷ��еȣ����ؿ���ѧ���ķ��������ͼ���������ע����Һ�е���غ��Ӧ�ú͵��볣���ļ��㷽����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
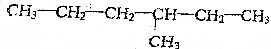
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ռ���� | B�� | �����¶� | ||
| C�� | ������������ | D�� | �����������ƹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
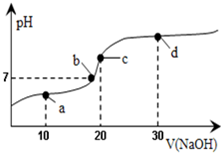 20��ʱ��20mL0.1mol/L������Һ�в��ϵ���0.1mol/LNaOH��aq������ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ������ǣ�������
20��ʱ��20mL0.1mol/L������Һ�в��ϵ���0.1mol/LNaOH��aq������ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ������ǣ�������| A�� | a�㣺c��CH3COO-����c��Na+����c��H+����c��OH-�� | B�� | b�㣺c��Na+��=c��CH3COO-����c��H+��=c��OH-�� | ||
| C�� | c�㣺c��H+��=c��CH3COOH��+c��OH-�� | D�� | d�㣺c��Na+����c��CH3COO-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
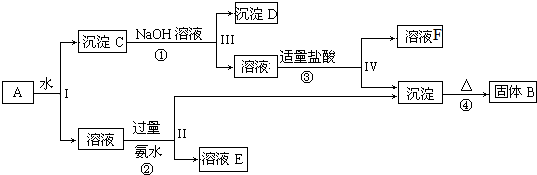
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO2+H2O | B�� | Cl2+H2O | C�� | Na+H2O | D�� | SO2+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuO | B�� | FeC13 | C�� | CuS | D�� | FeS |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com