
| �������ʵ | ��Ҫԭ�� | |
| A | ȼú����������CaO�ɼ���SO2�ŷ��� | ȼ������Ԫ��ת��ΪCaSO3 |
| B | ������ϩ��Ĥ�������ڰ�װʳƷ | ����ɰ�ɫ��Ⱦ |
| C | �����������о��������ر������ | ������O3�������ӡ��������� |
| D | �����Ŵ�����ʵ�������ת��Ϊ����ɿڵ���� | ���ֵ���ˮ���������������Ҵ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��ȼ�պ���Ԫ���������ơ�������Ӧ����CaSO4��
B������ϩ�ж����������ڰ�װʳƷ��
C�������������״�������ת���ɳ�����
D������ˮ�����������ǣ���������һ��������ת�����Ҵ��Ͷ�����̼��
��� �⣺A����ú�в�����ʯ�ң����������뷴Ӧ���µ������£���������SO2��������ƣ����Լ���SO2���ŷţ���A����
B��������ϩ��Ĥ�������ڰ�װʳƷ��ԭ���Ǿ�����ϩ�ж���������Ϊ��ɰ�ɫ��Ⱦ����B����
C������������������O3�������ӡ��������٣����Ըо��������ر�����£���C��ȷ��
D�������Ŵ�����ʵ�������ת��Ϊ����ɿڵ���ƣ�������һ��������ת���������ǣ������ǽ�һ��ת�����Ҵ�����D����
��ѡC��
���� ���⿼�������ʵ���ɡ��ṹ�����ʵĹ�ϵ����Ŀ�Ѷ��еȣ��漰����ϩ��������Ⱦ������������ˮ�⡢���������ת����֪ʶ����ȷ����������ɺ�����Ϊ���ؼ����������������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��������� | ���� | |
| A | ����Ba��OH��2 | ��Ӧ������c��CO32-����С |
| B | �ټ������Na2CO3 | ��Һ��c��CO32-����c��HCO3-�� |
| C | 100mLH2O | ��ˮ�������c��H+��•c��OH-������ |
| D | ������ | $\frac{c��C{O}_{3}^{2-}��}{c��HC{{O}_{3}}^{-}��}$���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
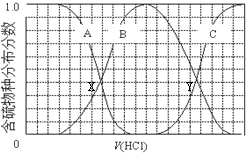
| A�� | ��������B��ʾHS- | |
| B�� | �ڵμ���������У���Һ��c��Na+���뺬�������Ũ�ȵĴ�С��ϵΪ��c��Na+��=3[c��H2S��+c��HS-��+c��S2-��] | |
| C�� | X��YΪ���ߵ�������㣬����֪��X�㴦��pH���Ϳ��Լ����H2S��Kaֵ | |
| D�� | NaHS�ʼ��ԣ�������Һ�м���CuSO4��Һ��ǡ����ȫ��Ӧ��������Һ��ǿ���ԣ���ԭ����Cu2++HS-�TCuS��+H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | �۵�/K | �е�/K | �ֽ��¶�/K |
| NH3 | 195.3 | 239.7 | 1073 |
| PH3 | 139.2 | 185.4 | 713.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʱ��/s | 0 | 2 | 4 | 6 | 8 | 10 |
| c��NO��/mol•L-1 | 1.00��10-3 | 4.50��10-4 | 2.50��10-4 | 1.50��10-4 | 1.00��10-4 | 1.00��10-4 |
| c��CO��/mol•L-1 | 3.60��10-3 | 3.05��10-3 | 2.85��10-3 | 2.75��10-3 | 2.70��10-3 | 2.70��10-3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������չ��̵�β�����պ������������ | |
| B�� | ���������У���6molCuFeS2��ȡ6molCuʱ������15molO2 | |
| C�� | �ڷ�Ӧ2Cu2O+Cu2S=6Cu+SO2���У����������뻹ԭ��������ʵ�����1��6 | |
| D�� | �ڷ�Ӧ2Cu2O+Cu2S=6Cu+SO2���У�ֻ��Cu2O�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
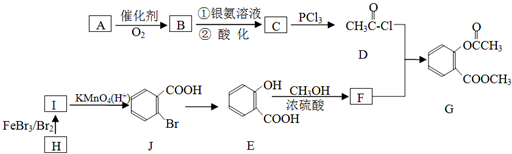
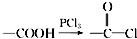 ����ش��������⣺
����ش��������⣺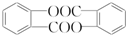 ��
��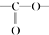 �ṹ
�ṹ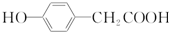 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | 25��ʱ��10-3mol/L�������pH=3��NH4Cl��Һ����pH=11�İ�ˮ�У�ˮ�ĵ���̶�Ϊ���ڣ��ۣ��� | |
| B�� | pH=1��NaHSO4��Һ��c��H+��=c��SO42-��+c��OH-�� | |
| C�� | pH=8.0��KHS��Һ�У�c��K+����c��HS-����c��OH-����c��S2-����c��H+�� | |
| D�� | ͼ��a����Һ�и�����Ũ�ȵĹ�ϵ�ǣ�c��OH-��=c��H+��+c��CH3COO-��+c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25��ʱ��pH=13�İ�ˮ��NaOH��Һ��1.0 L���е�OH-��Ŀ��Ϊ0.1NA | |
| B�� | ���³�ѹ�£���CO2��O2��ɵĻ�����й���NA�����ӣ����е���ԭ����Ϊ2NA | |
| C�� | 22 gD218O�����У��������������������ֱ�Ϊ10NA��12NA | |
| D�� | ��״���£�22.4 L CCl4�к��е�C-Cl����ĿΪ4NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com