
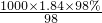 mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬����ҪŨ��������ΪV����
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬����ҪŨ��������ΪV����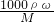 ����Ũ��������ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬������Ҫ��Ũ����������
����Ũ��������ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬������Ҫ��Ũ���������� �ж϶�������ҺŨ�ȵ�Ӱ�죮
�ж϶�������ҺŨ�ȵ�Ӱ�죮 ������Һ����ԭ������������
������Һ����ԭ������������ 

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ�����е�һ��ѧ����1���¿������ۣ���ѧ���� ���ͣ������
ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
��1����Ҫ��ȡ98%�ܶ�Ϊ1��84g/cm 3��Ũ���� mL[��Դ:ѧ����ZXXK]
3��Ũ���� mL[��Դ:ѧ����ZXXK]
��2������ʱ������ʹ�õ������� ������ ���� ������ţ�
���ձ� ����100 mL��Ͳ ������20 mL��Ͳ ��1000 mL����ƿ
��250 mL����ƿ�� ��������ƽ�������룩 �߲�����
��ȱ�ٵ������� ������ ��
��3������ʱ����ʵ�������õ����������������� ��
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
��û��ϴ���ձ��Ͳ���������������������������
�������ˮ�����˿̶��ߣ�����ˮʹҺ��ǡ�õ��̶��ߡ����������� ������
������ƿ��ϴ���û�и������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ��������������ѧ������ѧ���Ի�ѧ���� ���ͣ�ʵ����
��8�֣�ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
��1����Ҫ98%�ܶ�Ϊ1.84g/cm3��Ũ���� mL
��2������ʱ������ʹ�õ������� ���������� (�����)
���ձ�����100 mL��Ͳ������20 mL��Ͳ ��1000 mL����ƿ ��250 mL����ƿ����������ƽ(������) �߲����� ��ȱ�ٵ������� ������ ��
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� ��
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
��û��ϴ���ձ��Ͳ���������������������������
�������ˮ�����˿̶��ߣ�ȡ��ˮʹҺ��ǡ�õ��̶��ߡ�����������������
������ƿû�и������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�����и���1���¿������ۣ���ѧ���� ���ͣ�ѡ����
ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
��1����Ҫ��ȡ98%�ܶ�Ϊ1��84g/cm3��Ũ���� mL[��Դ:ZXXK]
��2������ʱ������ʹ�õ������� ������ ���� ������ţ�
���ձ� ����100 mL��Ͳ ������20 mL��Ͳ ��1000 mL����ƿ
��250 mL����ƿ�� ��������ƽ�������룩 �߲�����
��ȱ�ٵ������� ������ ��
��3������ʱ����ʵ�������õ����������������� ��
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
��û��ϴ���ձ��Ͳ������������������������� ��
�������ˮ�����˿̶��ߣ�����ˮʹҺ��ǡ�õ��̶��ߡ����� ������ ������
������ƿ��ϴ���û�и������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭��������ѧ��ѧ������ѧ�ڵ�һ�ν��Բ��Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
��1����Ҫ98%�ܶ�Ϊ1��84g/cm3��Ũ���� mL��1�֣�
��2������ʱ������ʹ�õ������� ���������� ������ţ���1�֣�
���ձ�����100 mL��Ͳ������20 mL��Ͳ ��1000 mL����ƿ ��250 mL����ƿ��������ƽ�������룩 �߲�����;��ȱ�ٵ������� ������ ����1�֣�
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� �� (��1��)
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�
����Ӱ �족��
��û��ϴ���ձ��Ͳ������������������������� ����1�֣�
�������ˮ�����˿̶��ߣ�ȡ��ˮʹҺ��ǡ�õ��̶��ߡ����� ��������������1�֣�
������ƿû�и��������������������1�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com