
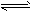 Na2S(s)+4H2O(g)����H<0��
Na2S(s)+4H2O(g)����H<0��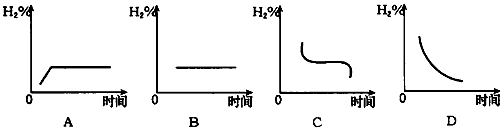
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�༶ͬѧ�������ϣ���Na2S����һ������ʶ������������¼������⣬��ش�
(1)1000��ʱ�����ܱ������м���һ������Na2SO4����ʹ�䷢�����·�Ӧ�ﵽƽ�⣺
Na2SO4(s)��4H2(g) ![]() Na2S(s)��4H2O(g)����H>0����Ӧ�ں��º���״̬�½��У��ش��������⣺
Na2S(s)��4H2O(g)����H>0����Ӧ�ں��º���״̬�½��У��ش��������⣺
�������зֱ�����������ʣ��ж϶�ƽ������Ӱ�죬��Ӱ�����д��ƽ���ƶ��ķ���
�ټ���Na2SO4�������������ڼ��뽹̿������������������������
(2)��Na2S��Ũ��Һ����μ���ϡ���ᣬֱ����������H2S����Ϊֹ�����ڴ˹����У���Һ��c(HS��)�仯���ƿ�������������
a.��С b.������
c.�����������С d.����С����������
(3)Na2S��Һ��������Һ��ϣ����ܷ�����Ӧ��������������
��H2S����SO2����Na2SO3��������KMnO4��Һ����CuSO4 ����ˮ
(4)д��Na2S��AlCl3��Һ��Ӧ�����ӷ���ʽ��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�༶ͬѧ�������ϣ���Na2S����һ������ʶ������������¼������⣬��ش�
(1)1000��ʱ�����ܱ������м���һ������Na2SO4����ʹ�䷢�����·�Ӧ�ﵽƽ�⣺
Na2SO4(s)��4H2(g) Na2S(s)��4H2O(g)����H>0����Ӧ�ں��º���״̬�½��У��ش��������⣺
�������зֱ�����������ʣ��ж϶�ƽ������Ӱ�죬��Ӱ�����д��ƽ���ƶ��ķ���
�ټ���Na2SO4�������������ڼ��뽹̿������������������������
(2)��Na2S��Ũ��Һ����μ���ϡ���ᣬֱ����������H2S����Ϊֹ�����ڴ˹����У���Һ��c(HS��)�仯���ƿ�������������
a.��С b.������
c.�����������С d.����С����������
(3)Na2S��Һ��������Һ��ϣ����ܷ�����Ӧ��������������
��H2S����SO2����Na2SO3��������KMnO4��Һ����CuSO4 ����ˮ
(4)д��Na2S��AlCl3��Һ��Ӧ�����ӷ���ʽ��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ��ɳ��һ��ѧ�����������¿���ѧ�Ծ� ���ͣ������
ij�༶ͬѧ�������ϣ���Na2S����һ������ʶ������������¼������⣬��ش�
(1)1000��ʱ�����ܱ������м���һ������Na2SO4����ʹ�䷢�����·�Ӧ�ﵽƽ�⣺
Na2SO4(s)��4H2(g)  Na2S(s)��4H2O(g)����H>0����Ӧ�ں��º���״̬�½��У��ش��������⣺
Na2S(s)��4H2O(g)����H>0����Ӧ�ں��º���״̬�½��У��ش��������⣺
�������зֱ�����������ʣ��ж϶�ƽ������Ӱ�죬��Ӱ�����д��ƽ���ƶ��ķ���
�ټ���Na2SO4�������������ڼ��뽹̿������������������������
(2)��Na2S��Ũ��Һ����μ���ϡ���ᣬֱ����������H2S����Ϊֹ�����ڴ˹����У���Һ��c(HS��)�仯���ƿ�������������
a.��С b.������
c.�����������С d.����С����������
(3)Na2S��Һ��������Һ��ϣ����ܷ�����Ӧ��������������
��H2S����SO2����Na2SO3��������KMnO4��Һ����CuSO4 ����ˮ
(4)д��Na2S��AlCl3��Һ��Ӧ�����ӷ���ʽ��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�����ظ�����ѧ�߶���һ���¿���ѧ�Ծ����������� ���ͣ������
1000��ʱ�����ܱ������м���һ������Na2SO4����ʹ�䷢�����·�Ӧ����ƽ�⣺Na2SO4(s)+4H2(g) Na2S(s)+4H2O(g)����H<0���ں��º���ʱ�ش��������⣺
Na2S(s)+4H2O(g)����H<0���ں��º���ʱ�ش��������⣺
(1)�������зֱ�����������ʣ��ж϶�ƽ������Ӱ�죬��Ӱ���Ӧ��д��ƽ���ƶ��ķ���
�ټ���Na2SO4_________________���ڼ��뽹̿________________��
(2)����ʱ�����Na2SO4��1��42g��ƽ��ʱ�����й�������������1��10g��Na2SO4��ת������_______________��
(3)���������¶�����20�棬H2�ڻ�������к����仯����ͼ��_____ ͼ��ʾ����ʡ�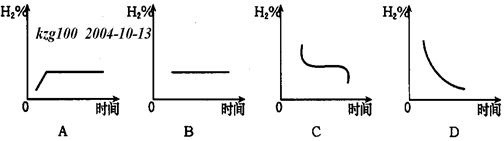
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�찲��ʡ�߶���һ���¿���ѧ�Ծ��������棩 ���ͣ������
1000��ʱ�����ܱ������м���һ������Na2SO4����ʹ�䷢�����·�Ӧ����ƽ�⣺Na2SO4(s)+4H2(g) Na2S(s)+4H2O(g)����H<0���ں��º���ʱ�ش��������⣺
Na2S(s)+4H2O(g)����H<0���ں��º���ʱ�ش��������⣺
(1)�������зֱ�����������ʣ��ж϶�ƽ������Ӱ�죬��Ӱ���Ӧ��д��ƽ���ƶ��ķ���
�ټ���Na2SO4_________________���ڼ��뽹̿________________��
(2)����ʱ�����Na2SO4��1��42g��ƽ��ʱ�����й�������������1��10g��Na2SO4��ת������_______________��
(3)���������¶�����20�棬H2�ڻ�������к����仯����ͼ��_____ ͼ��ʾ����ʡ�
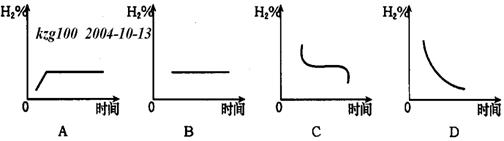
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com