
��ѧ�������֮����ת�����˵�����ʵ��������أ�������������������Ҫ��Ӧ�ã�ͬʱҲ��ѧ���γɻ�ѧѧ����������Ҫ��ɲ��֡�
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ����(��ͼ1)����Ӧԭ��Ϊ��2Na��FeCl2 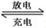 Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
���ʱ��__________(д��������)�缫�ӵ�Դ�ĸ�����
�õ�صĵ����Ϊ________ _��
��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ(��ͼ2)��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺
��Y�缫������ ������ (�������ԭ��)��Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫����Ӧʽ�ǣ�
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������
��3������ʱ��BaSO4��Ksp��1.08��10-10,�ֽ��������BaCl2��Һ��2.0��10-3mol/l��Na2SO4
��Һ��ϡ���Ҫ����BaSO4������BaCl2��Һ����СŨ��Ϊ______________��
(14��)
��1��Fe2��+2e��= Fe��2�֣����ƣ�1�֣����¡�Al2O3��1�֣�
��2����ʯī��1�֣���������1�֣�
��Cu2����2e��=Cu��2�֣���2H����2e��=H2����2H2O��2e��=H2����2OH����2�֣�
��0.448L��448mL��2�֣�û�е�λ��1��
��3��2.16x10-7. ��2�֣�
������������� ��1����������ԭ��Ӧ�������ϼ۽��͵�Ԫ�ط�����ԭ��Ӧ�����������ʱ���������������ܵ�������������Һ��
��2���ɼ���0.98g������ͭ����ȫ�ܽ⣬������Һ����ǰ��ȫ��ͬ����֪��Һ���������ͭ���������ŵ����Cu2�����������ŵ����OH-��С�մ�NaHCO3��
Cu SO4 + 2 H2O= Cu���ϣȣ�����H2SO4��n=0.98g/98gmol-1=0.01mol
��NaHCO3��H2SO4��Na��SO4��H2O����CO������������
0.01mol 0.02mol
0.02mol��22.4L/mol=0.448L
��3�� �������BaCl2��Һ��2.0��10-3mol/l��Na2SO4��Һ���
Ksp���㣨Ba2�����㣨SO4���������㣨Ba2������.0��10-3��1.08��10-10
�㣨Ba2������1.08��10-7����ԭ��Ba2����=2.16x10-7.
���㣺���⿼�����֪ʶ��Ksp�ļ����֪ʶ��


 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��25��ʱ��ijNaCl��Һ��c(Cl�C)��1��10��4 mol��L�C1�������Һ��c(Na��)��c(OH��)��
��2��25��ʱ����0.1 mol��L�C1NaOH��Һ��0.06 mol��L�C1��H2SO4��Һ��������(���Ի�Ϻ�����ı仯)����������Һ��pH�� ��25��ʱ��pHֵΪ8��NaOH��Һ��pHֵΪ10��NaOH��Һ�������Ϻ���Һ��������Ũ����ӽ� ��
��3��25��ʱ������������Һ�У���pH=0������ ��0.1 mol��L�C1������ ��0.01 mol��L�C1��NaOH��Һ ��pH=11��NaOH��Һ����ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U���ǣ� (����ĸ)
| A��1�U10�U100�U1000 | B��0�U1�U12�U11 |
| C��14�U13�U12�U11 | D��14�U13�U2�U3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��17�֣���ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�
��1��һ�������£�ģ��ij��ʯ�γɵķ�ӦaW+bQ��cN+dP+eR�õ�����ͼ��
�ٸ÷�Ӧ�ġ�H 0���>������=����<������
��ij�¶��£�ƽ�ⳣ������ʽΪK =c2��X��������ͼ��2���ж�X����������Ϊ____��
��2����E��F�����ܱ������У���һ�������·�����Ӧ��E��g��+F��s�� 2G��g��������
2G��g��������
���������ƽ��ʱG�����������%�����¶Ⱥ�ѹǿ�ı仯���±���ʾ��
��K��915�棩��K��810�棩�Ĺ�ϵΪK��915�棩____K��810�棩������ڡ��������ڡ���С�ڡ�����a��b��f���ߵĴ�С��ϵΪ ��1000�桢3��0 MPaʱE��ת����Ϊ____����3��25��ʱ��H2CO3 HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O
HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O  HCO3��+OH������ʵ ��
HCO3��+OH������ʵ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��֪��Cu(OH)2�Ƕ�Ԫ��������ᣨH3PO3���Ƕ�Ԫ���ᣬ��NaOH��Һ��Ӧ������Na2HPO3��
��1����ͭ����Һ��Cu2������ˮ�ⷴӦ�����ӷ���ʽΪ____���÷�Ӧ��ƽ�ⳣ��Ϊ____������֪��25��ʱ��Ksp[Cu(OH)2]��2.0��10��20mol3/L3��
��2������H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH______7��������������������������£���10mL0.01mol/L H3PO3��Һ�еμ�10ml0.02mol/LNaOH��Һ����Һ�и�������Ũ���ɴ�С��˳����_________��
��3�����Na2HPO3��Һ�ɵõ������ᣬװ����ͼ��˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����
�������ĵ缫��ӦʽΪ____________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��17�֣������ҹ���ҵ��ˮƽ�IJ��Ϸ�չ�����ˮ��������Ⱦ�����Ϊ��Ҫ���⡣
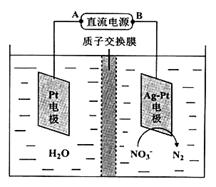
��1������β���Ĵ����ŷ�����ɿ�����Ⱦ����Ҫ����֮һ����չȼ�ϵ������������Ч�ؽ���������⡣ֱ�Ӽ״�ȼ�ϵ��(DMFC)��������к��������ת��Ч�ʱ���ȼ��Ҫ��2��3������ؽṹ��ͼ��ʾ��c��ͨ�������ΪΪ______�����·�е��Ӵ�______��______(�A����B��)�ƶ���д����ظ����ĵ缫��Ӧ����ʽ 
��2����ҵ��ˮ�г�����һ������Cr2O72����������༰��̬ϵͳ�����ܴ�����ⷨ�Ǵ�������Ⱦ�ij��÷������÷���Fe���缫��⺬Cr2O72�������Է�ˮ�����ʱ�����������д����������ɣ�������Cr(OH)3��Fe(0H)3������
�ٷ�Ӧ�У�1molCr2O72����ȫ����Cr(OH)3���������·ͨ�����ӵ����ʵ���Ϊ_________ mol��
�ڳ����£�Cr(OH)3���ܶȻ� ����Cr3��Ũ��С��10
����Cr3��Ũ��С��10 mol
mol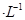 ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
��3��������ˮ������ˮ�帻Ӫ��������NH4Cl��Һ�м�������NaOH���壬��Һ�� ________���������С�����䡱����25
________���������С�����䡱����25 ʱ��NH3?H2O�ĵ���ƽ�ⳣ��
ʱ��NH3?H2O�ĵ���ƽ�ⳣ�� �����¶���,1mol
�����¶���,1mol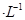 ��NH4Cl��Һ��
��NH4Cl��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ᡢ����ǻ�ѧ�������о�����Ҫ������ش����и�С�⣺��1��ij��Ԫ��H2X�ĵ��뷽��ʽ�ǣ�H2X=H++HX����HX��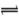 X2��+H+���ش��������⣺
X2��+H+���ش��������⣺
��KHX��Һ�� ������ԡ��������ԡ������ԡ�����
����0.1 mol��L��1KHX��Һ��pH=2����0.1 mol��L��1H2X��Һ�������ӵ����ʵ���
Ũ�� �����������������=����0.11 mol��L��1�������� ��
��0.01 mol��L��1��HCl��0.02 mol��L��1��KHX��Һ��������Һ�и�����Ũ���ɴ�С��˳����  ��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4��
��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4��
��3����25���£���pH��3�Ĵ�����Һ��pH��11������������Һ����������������Һ�����ʵ���Ũ���� ��������Һ�����ʵ���Ũ�� �����������������������������������Һ���ʵ���Ũ�ȡ�����������Һ�������ϣ���Ӧ����Һ�� ����ᡱ����������С����ԡ�
��4��ijǿ���Է�Ӧ��ϵ�У�������Ӧ��
X+ PbO2+ H2SO4 = Pb��MnO4��2+ PbSO4+ H2O ��
��֪X��һ�������Σ���0.2 mol X�ڸ÷�Ӧ��ʧȥ1 mol ���ӣ���X�Ļ�ѧʽ��
���뽫������ѧ����ʽ��ƽ����ϵ�����ڸ�����ǰ�ĺ����ϡ�.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʳ�ð״����ɴ����봿ˮ���ƶ��ɣ����к͵ζ��ķ���ȷ�ⶨ���д�������ʵ���Ũ�ȡ�ʵ�鲽�裺������500mLŨ��ԼΪ0��1mol��L-1��NaOH��Һ������KHC8H4O4����Һȷ�ⶨ��NaOH��Һ��Ũ�ȣ�������֪ȷŨ�ȵ�NaOH��Һ�ⶨ�����Ũ�ȡ�
��1�����������NaOH�������ڴ��ձ��У�����500mL����ˮ�������ܽ⡣�����Ʋ��� ������С������С�����
��2������ʱNaOH�ڿ����м�����ˮ���������õ�NaOH��ҺŨ��ͨ����Ԥ�� ���С���������Dz���ֱ�����������Һ��ԭ��
��3�����İ״װ�װ�����Ậ��ԼΪ6g/100mL����������ʵ�Ũ��ԼΪ mol��L-1���ζ�ǰҪ�Ƚ��״�ϡ��10����ϡ�Ͱ״�ʱ��Ҫ��������100mL����ƿ���ձ����������� ��ͷ�ιܡ� ��
��4��ȷ��ȡϡ�ͺ�İ״�20��00mL������250mL��ƿ�У�����30mL����ˮ���ٵμӷ�ָ̪ʾ����������NaOH����Һ�ζ��� ��Ϊ�յ㡣
��5��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������Ϊ20��00mL��NaOH��ҺŨ��Ϊc mo1/L������ʵ ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 25��02 | 24��22 | 24��18 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ƣ�NaNO2������Ҫ�ķ�������ij��ѧ��ȤС�鳢���Ʊ��������ƣ��������ϣ���HNO2Ϊ���ᣬ��������Һ�У�NO2-�ɽ�MnO4-��ԭΪMn2�������������ɡ�
��NO����Ӧ���ɱ�����KMnO4��Һ����Ϊ����
̽��һ �������ƹ�����Ʊ�
��̼��Ũ����Ϊ��ʼԭ�ϣ��������װ������һ��������������Ʒ�Ӧ�Ʊ��������ơ�����Ӧ����ʽΪ2NO��Na2O2=2NaNO2�����ּг�װ�ú�A�м���װ�����ԣ�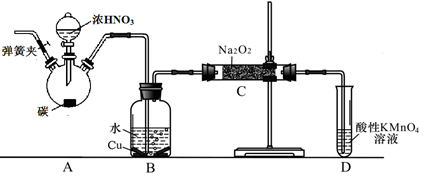
��1��д��װ��A��ƿ�з�����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊװ��C�в��ﲻ�����������ƣ�����̼���ƺ��������ƣ�Ϊ�ų�����Ӧ��B��Cװ�ü�����װ��E��E��ʢ�ŵ��Լ�Ӧ�� ������ĸ����
A��ŨH2SO4 B����ʯ�� C����ˮCaCl2
̽���� �������ƹ��庬���IJⶨ��������֤
��ȡװ��C�з�Ӧ��Ĺ���4.000g����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000
mol/L����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4��Һ���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ͼ��ʾ����пƬ��ͭƬ�õ������������װ��ϡ������Һ���ձ��й���ԭ��ء�����������ȷ����
| A��Zn�Ǹ�����������ԭ��Ӧ |
| B��������пƬ����ͭƬ |
| C��һ��ʱ���ͭƬ�������� |
| D����װ�ý���ѧ��ת��Ϊ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com