
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2(7.8%)��Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ����ù������£�

(ע��SiCl4�ķе�Ϊ57.6 �棬�����Ȼ���ķе������150 ��)
(1)��Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ��N2����ҪĿ����____________________��
(2)���·�Ӧ��ʯī�����������ʾ�ת��Ϊ��Ӧ���Ȼ��������е�̼��������ҪΪ________�����������ij��õ�ˮ�����Ļ�ѧ��Ӧ����ʽΪ____________________________________________��
(3)�����Ϊ�����衢________��������Һ���е���������________��
(4)����Һ�����ɳ��������ܷ�Ӧ�����ӷ���ʽΪ______________________________________________��100 kg����ʯī�����ܻ�â�������Ϊ______kg��
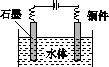
(5)ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��
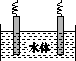
(1)�ų�װ���еĿ��� ��
(2)CO�� SiCl4��6NaOH===Na2SiO3��4NaCl��3H2O ��
(3)���ˡ�AlO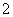 ��Cl�� ��
��Cl�� ��
(4)AlO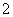 ��CH3COOCH2CH3��2H2O
��CH3COOCH2CH3��2H2O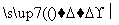 CH3COO����CH3CH2OH��Al(OH)3����7.8 ��
CH3COO����CH3CH2OH��Al(OH)3����7.8 ��
(5)
[����] (1)ͨ��N2��Ŀ����Ϊ���ų�װ���еĿ���(�ر�������)����ֹ�ڸ���ʱʯī��������Ӧ��(2)���·�Ӧ��SiO2��Al2O3��Fe2O3��MgO�ֱ�ת��ΪSiCl4��AlCl3��FeCl3��MgCl2����Ϊʯī�ǹ����ģ��ʸ��������£�C��SiO2��Fe2O3��Ӧ���ɵ���CO������SiCl4�ķе�Ϊ57.6 �棬����80 �棬�������ΪSiCl4����NaOH��Һ��ˮ������Na2SiO3��NaCl: SiCl4��6NaOH===Na2SiO3��4NaCl��3H2O��(3)AlCl3��FeCl3��MgCl2�ķе������150 �棬��80 ���±�Ϊ����� AlCl3��FeCl3��MgCl2����NaOH��Ӧ������NaAlO2��Fe(OH)3��Mg(OH)2��NaCl��ͨ�����˽�����Fe(OH)3��Mg(OH)2�˳����õ�����Һ����Ҫ��NaAlO2��NaCl��(4)NaAlO2����ˮ�����Һ�Լ��ԣ�NaAlO2��2H2O Al(OH)3��NaOH������������������ˮ�⣺CH3COOCH2CH3��NaOH
Al(OH)3��NaOH������������������ˮ�⣺CH3COOCH2CH3��NaOH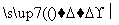 CH3COONa��CH3CH2OH����ʹNaAlO2����ˮ������Al(OH)3��������Һ��ת��Ϊ�������ķ�ӦΪNaAlO2��2H2O��CH3COOCH2CH3
CH3COONa��CH3CH2OH����ʹNaAlO2����ˮ������Al(OH)3��������Һ��ת��Ϊ�������ķ�ӦΪNaAlO2��2H2O��CH3COOCH2CH3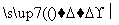 Al(OH)3����CH3COONa��CH3CH2OH������Alԭ���غ㣬��֪100 kg����ʯī�ɵ�m[Al(OH)3]��
Al(OH)3����CH3COONa��CH3CH2OH������Alԭ���غ㣬��֪100 kg����ʯī�ɵ�m[Al(OH)3]��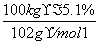 ��2��78 g��mol��1��7.8 kg��
��2��78 g��mol��1��7.8 kg��
(5)ˮ����ͭ���ĵ绯ѧ���������˵�Ᵽ�������÷�����ʯī��������ͭ��������������ӵ���������������������ʯī��ͭ��ֱ���������γ�ԭ��أ���ͭ�����������������Բ��ɲ��á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������Ҫ��������������������й㷺Ӧ�á����������ж����������γ��������Ҫ���塣������ʵ�����Ʊ����ǹ�ҵ��������������β�����ջ��������dz���Ҫ��
���������գ�
ʵ���ҿ���ͭ��Ũ������Ȼ�������������Ʒ�Ӧ��ȡ��������
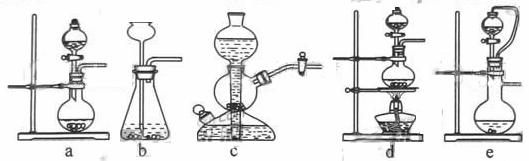
�����������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ���ͼ�п�ѡ�õķ���װ���� ����д��ĸ����
41.����������������Ʒ�Ӧ��ȡ3.36L����״������������������Ҫ��ȡ��������
g������һλС�������������4.0%�������ƣ��������������������������ƣ����������ȡ���������� g ������һλС������
42.ʵ���Ҷ�������β�������빤ҵ��������Ļ�ѧԭ����ͨ��ʯ��-ʯ�෨�ͼ�dz��õ���������
ʯ��-ʯ�෨�����շ�ӦΪSO2+Ca(OH)2��CaSO3��+H2O�����ղ�����������ɹܵ���������������������ӦΪ2CaSO3+O2+4H2O��2CaSO4��2H2O������������ͼ��
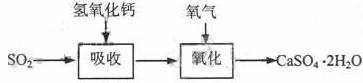
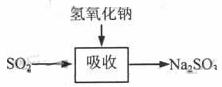
������շ�ӦΪSO2+2NaOH��Na2SO3+H2O������ص����������Ƽ���ǿ�����տ졢Ч�ʸߡ�����������ͼ��
��֪��
| �Լ� | Ca(OH)2 | NaOH |
| �۸�Ԫ/kg�� | 0.36 | 2.90 |
| ����SO2�ijɱ���Ԫ/mol�� | 0.027 | 0.232 |
ʯ��-ʯ�෨�ͼ���ն�������Ļ�ѧԭ����֮ͬ���� ���ͼ��ȣ�ʯ��-ʯ�෨���ŵ��� ��ȱ���� ��
43.��ʯ��-ʯ�෨�ͼ�Ļ����ϣ����һ���Ľ��ġ���ʵ������ѭ��������������������ͼ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʯ����Ҫ�ɷ���CaCO3��MgCO3�����ҹ��д����ķֲ�������ʯΪԭ�������ĸ�þϵ�в�Ʒ�й㷺����;������ʯ�����ա��ۻ���õ���þ����������پ���̼��ʵ��Ca2+��Mg2+�ķ��롣̼����Ӧ�Ƿ��ȷ�Ӧ����ѧ����ʽ���£�Ca(OH)2 + Mg(OH)2 + 3CO2⇌ CaCO3 + Mg(HCO3)2 + H2O
����������
Ca(OH)2�ļ��Ա�Mg(OH)2�ļ��� ��ѡ�ǿ����������
Ca(OH)2���ܽ�ȱ�Mg(OH)2���ܽ�� ��ѡ���С����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ч���ٶ���������ŷš�ʵ�����÷�ú��(��Ҫ��Al2O3��SiO2��)�Ʊ���ʽ������[Al2(SO4)x(OH)6��2x]��Һ�����������������о���

(1)���ʱ��Ӧ�Ļ�ѧ����ʽΪ____________________�����������Ҫ�ɷ�Ϊ________(�ѧʽ)��
(2)��CaCO3������Һ��pH��3.6����Ŀ�����к���Һ�е��ᣬ��ʹAl2(SO4)3ת��ΪAl2(SO4)x(OH)6��2x�����������Ҫ�ɷ�Ϊ________(�ѧʽ)������Һ��pHƫ�ߣ����ᵼ����Һ����Ԫ�صĺ������ͣ���ԭ����__________________________(�����ӷ���ʽ��ʾ)��
(3)���������о���ȫ�ȷֽ�ų���SO2������С�����յ�SO2��������Ҫԭ����________________________��������SO2ǰ����Һ��ȣ��ȷֽ��ѭ�����õ���Һ��pH��________(�������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У���Ӧ������������ص���(����)
A������ͨ�����ȵ�CuO��ĩ
B��������̼ͨ��Na2O2��ĩ
C������Fe2O3�������ȷ�Ӧ
D����п��Ͷ��Cu(NO3)2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������л�ѧ������˵����ȷ����(����)
��������������������������������
A�����·������������ۻ�Ϊͬ���칹��
B���Ʒ��ס����С���ѹ���ȵIJ�����ǺϽ�
C����ըʳ��Ļ����ͺ�ţ�Ͷ��ǿ������ı�������
D��ĥ�����Ĵ��������ʣ�������к��ʱ���˰�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1.52g ͭþ�Ͻ���ȫ�ܽ���50mL �ܶ�Ϊ1.40 g/mL����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������1120 mL����״��������Ӧ�����Һ�м���1.0 mol/L NaOH��Һ������������ȫ������ʱ���õ�2.54 g����������˵������ȷ���ǣ�������
A���úϽ���ͭ��þ�����ʵ���֮����2:1
B. ��Ũ������HNO3�����ʵ���Ũ����14.0 mol/L
C��NO2��N2O4�Ļ�������У�NO2�����������80%
D���õ�2.54 g����ʱ������NaOH��Һ�������600 mLzxxk
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǵؿ��к�����ߵĽ���Ԫ�أ��䵥�ʼ��Ͻ������������е�Ӧ�������㷺��
(1)���̼�Ȼ�ԭ-�Ȼ�����ʵ���������Ʊ�������������ص��Ȼ�ѧ����ʽ���£�
Al2O3(s)��AlCl3(g)��3C(s)=3AlCl(g)��3CO(g) ��H=a kJ��mol��1
3AlCl(g)=3Al(l)��AlCl3(g) ��H=b kJ��mol��1
�ٷ�ӦAl2O3(s)��3C(s)=2Al(l)��3CO(g)�ġ�H= kJ��mol��1(�ú�a��b�Ĵ���ʽ��ʾ)��
��Al4C3�Ƿ�Ӧ���̵��м���Al4C3�����ᷴӦ(����֮һ�Ǻ�������ߵ���)�Ļ�ѧ����ʽ ��
(2)þ���Ͻ�(Mg17Al12)��һ��DZ�ڵ�������ϣ�������������£���һ����ѧ�����ȵ�Mg��Al������һ���¶���������á��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪ
Mg17Al12��17H2=17MgH2��12Al���õ��Ļ����Y(17MgH2��12Al)��һ���������ͷų�������
�������Ʊ�þ���Ͻ�(Mg17Al12)ʱͨ�������Ŀ���� ��
����6.0mol��L��1HCl��Һ�У������Y����ȫ�ͷų�H2��1 mol Mg17Al12��ȫ�����õ��Ļ����Y������������ȫ��Ӧ���ͷų�H2�����ʵ���Ϊ ��
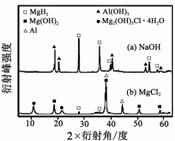 ����0.5 mol��L��1 NaOH��1.0 mol��L��1 MgCl2��Һ�У�
����0.5 mol��L��1 NaOH��1.0 mol��L��1 MgCl2��Һ�У�
�����Y��ֻ�ܲ��ַų���������Ӧ�������������X-����������ͼ����ͼ��ʾ(X-��������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ)��������NaOH��Һ�У������Y�в�����������Ҫ������
(�ѧʽ)��
(3)�����������Խ��Al-AgO��ؿ�����ˮ��
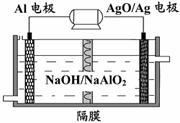 ������Դ����ԭ��������ͼ��ʾ���õ�ط�Ӧ
������Դ����ԭ��������ͼ��ʾ���õ�ط�Ӧ
�Ļ�ѧ����ʽΪ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
(1)��ԭ������������˳��(ϡ���������)������˵����ȷ����________��
a��ԭ�Ӱ뾶�����Ӱ뾶����С
b�������Լ������ǽ�������ǿ
c���������Ӧ��ˮ������Լ�����������ǿ
d�����ʵ��۵㽵��
(2)ԭ�������������������������ͬ��Ԫ������Ϊ________�������������ļ���������________��
(3)��֪��
| ������ | MgO | Al2O3 | MgCl2 | AlCl3 |
| ���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
| �۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����__________________________________��
����ʱ�����Al2O3�������AlCl3��ԭ����______________________________��
(4)�����(�۵�1410 ��)�����õİ뵼����ϡ��ɴֹ��ƴ���������£�
Si(��)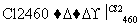 SiCl4
SiCl4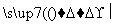 SiCl4(��)
SiCl4(��)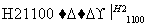 Si(��)
Si(��)
д��SiCl4�ĵ���ʽ��________________����������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12 kg����������a kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(5)P2O5�Ƿ������Ը�������������岻����Ũ����������P2O5�������________��
a��NH3 ��b��HI c��SO2 d��CO2
(6)KClO3������ʵ������O2�������Ӵ�����400 ��ʱ�ֽ�ֻ���������Σ�����һ�����������Σ���һ���ε��������Ӹ�����Ϊ1��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com