
| A��ֻ�Т� | B��ֻ�Т� | C���ٺ͢� | D���ں͢� |
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����CCl4��ȡ��ˮ�е����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| B�������У���ȴˮӦ�������ܵ��¿�ͨ�룬�Ͽ����� |
| C������ɫ��Ӧ����Na+ʱ���ò�����պȡ�������ڻ��������չ۲������ɫ |
| D�������ᾧʱ�������ȵ��д����������ʱֹͣ���ȣ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | A | B | C | D |
| ��ɢ��ˮ�� | ������Һ | ����ɫ��Һ | Һ��ֲ㣬���²�Ϊ��ɫ��״Һ�� | ����ɫ��Һ |
| �۵㣨�棩 | 1452 | ��21.3 | ��11.5 | 801 |
| �е㣨�棩 | 1703 | 78.9 | 117 | 1210 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ù��˵ķ�����ȥʳ��ˮ�е���ɳ | B�������Ȼ�̼��ȡ��ˮ�еĵ� |
| C��������ķ���������ˮ�Ƴ�����ˮ | D���÷�Һ�ķ������������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
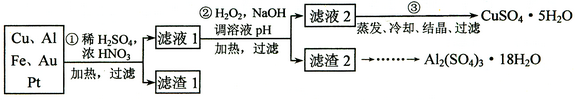
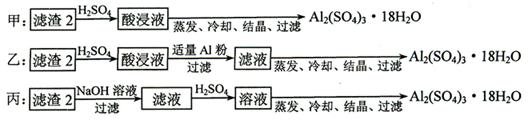
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| ���� | ������Լ� |
| CaCl2 | ��________ |
| MgCl2 | ��________ |
| ������ | ��________ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com