
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| �ζ����� | ����Һ���(mL) | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.50 | 25.45 |
| �ڶ��� | 20.00 | 4.00 | 29.05 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
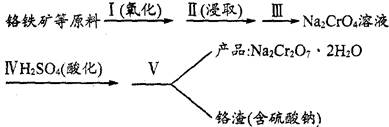
 8Na2CrO4(s)��2Fe2O3(s)��8CO2���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪ)_____________���ڳ����¸÷�Ӧ���ʼ��������д�ʩ�в���ʹ��Ӧ�����������____________ ��
8Na2CrO4(s)��2Fe2O3(s)��8CO2���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪ)_____________���ڳ����¸÷�Ӧ���ʼ��������д�ʩ�в���ʹ��Ӧ�����������____________ ��| ��� | ʵ�鲽�� | ����ʵ�����(�������������װʵ��װ��) |
| �� | �ܽ� | �����������ձ��С���ˮ����ֽ���ֱ�����岻���ܽ� |
| �� | | |
| �� | | |
| �� | | |
| �� | ���ˡ����� | �õ�K2Cr2O7���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A������������ϡ������Һ����ȡ����Ũ�������Թ��м�ˮϡ�� |
| B����������ƾ�ʧ��ȼ�գ�����ʪĨ��������� |
| C��������һ�ְ�ɫ�ľ��壬����Ϊ��̽��������ζ���ɴ��Լ�ƿ��ȡ��������Ʒ�� |
| D��Ϊ�˽�Լʵ��ҩƷ��ʵ��ʣ���ҩƷҪ�Ż�ԭƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����pH��ֽ������Һ�вⶨ��Һ��pH |
| B��Ϊ��ȥ���е���������,�������м�����������ˮ����� |
| C������մ��Ƥ���������þƾ���ϴ |
D����������ϴ�� ��������Ӧʵ����Թ� ��������Ӧʵ����Թ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ���������ᶡ��ʱ����ˮԡ���� |
| B���������ķ���������֬������Ӧ�IJ��� |
| C��ʵ��ʱ��ָ��С��մ�ϱ��ӣ�������70��������ˮ��ϴ |
| D��ʵ������������ʱ���������뱽��Ϻ��ٵμ�Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢܢݢ� | B���ڢۢܢ� | C���ڢۢݢ� | D���٢ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ư�۳��ڷ������ձ��� |
| B���������Ż�ȼ��ʱ����������ĭ��������Ӧ����ɳ����� |
| C����ˮ��������ɫ�����Լ�ƿʢװ���� |
| D���д���������й©ʱ��������������Һ��ʪ�������棬��Ѹ���뿪�ֳ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com