
������ͭ�Ǵ���ˮ�潢������Ϳ�����Ҫԭ�ϡ�ijС����������о�������д���пհס�
ʵ��1��������ͭ����ȡ������ͭ���������Ǻ�����������ͭ����Һ��Ӧ��ȡ�����ױ������������Ʋ���ʱ��������CuO���ɡ�
��1��ʵ������ȡ������ͭ����Һ�����ӷ���ʽΪ____________��
��2��ʵ�����ô˷�����ȡ���������������ͭ���壬��Ҫ�IJ����������Թܡ��ƾ��ơ��ձ�____________��____________��
��3����Ҫ̽���÷�Ӧ����������¶ȣ�Ӧѡ�õļ��ȷ�ʽΪ____________��
ʵ��2���ⶨ������ͭ�Ĵ���
����1����ȡʵ��1���ù���m g����������װ�ý���ʵ�顣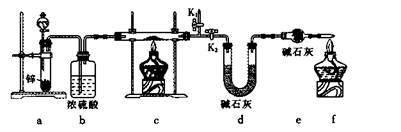
��4��װ��a�����ӵ�����____________���ѧʽ����
��5��ͨ������������������ܴﵽʵ��Ŀ�ĵ���____________��
| A����Ӧǰ��װ��a������ |
| B��װ��c��ַ�Ӧ�����ù�������� |
| C����Ӧǰ��װ��d������ |
| D����Ӧǰ��װ��e������ |
��1��Cu2++2OH��=Cu(OH)2����2�֣�
��2��©��������������1�֣���2�֣�
��3��ˮԡ���ȣ�2�֣�
��4��H2SO4��1�֣�
��5��BC��ѡ��1����ȫѡ����2�֣�
��6����K2���ر�K1��2�֣�
��7�����������ٴθ���������ֱ�������������������ͬ�������������𰸣���2�֣�
��8��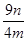 ��100% �������������𰸣���2�֣�
��100% �������������𰸣���2�֣�
���������������1��ʵ���ҳ��ÿ�����ͭ����Һ���������Ʒ�Ӧ��ȡ������ͭ����ӦʽΪCu2++2OH��=Cu(OH)2������2��������������������ͭ���ֱ�Ӽ�����Ҫ�Թܡ��ƾ��ƣ���Һ���з����Cu2O������Ҫ�ձ���©��������������3��̽���÷�Ӧ����������¶ȣ�����ѡ��ˮԡ���ȣ���Ҫ�¶ȼƲ���ˮԡ���¶ȣ���4��п����������ᡢ�ѻӷ����ᷴӦ������ȡ��������aװ��Ӧ����ϡH2SO4����5��װ��d���ӵ�����Դ��c�з�Ӧ������ˮ���ɴ˿��Լ������Ʒ����Ԫ�ص�������c��Ӧ�����ù������������ͭ��������������������Ԫ�ص��������Բ��������ͭ�Ĵ��ȣ���BC��ȷ����6����K2���ر�K1��ͨ����һ������ټ���cװ�ã���7�����������ٴθ���������ֱ�������������������ͬ�������������𰸣���˵���������Ƿ�����ȫ�����8����m/M��֪n(Cu)=n/64mol����Cu2O+2H+=Cu2++Cu+H2O��֪n(Cu2O)=n/64mol����n?M��֪m(Cu2O)=144n/64g=9n/4g������Ʒ��Cu2O�Ĵ���Ϊ9n/4m��100%��
���㣺���������Ʊ�ʵ�顢������Ʒ�Ĵ��ȡ�����������ᴿ�ķ��������ʵļ��ȡ���ѧ��������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʽ���ý�ѧ���о���ѧϰ��һ�ַ�ʽ���������ѧģʽΪ��
��ͼ���ǹ��ڡ�һ����̼�Ļ�ѧ���ʡ��Ŀ���ʽ���ý�ѧ�н������Σ���ͬѧ��Ƶ�֤��CO���л�ԭ�Ե�ʵ��װ�á�
��
(1)ʵ��ʱӦ�ȵ�ȼ_____________(�A����B��)���ľƾ��ơ�
(2)Ӳ�ʲ������з�Ӧ�Ļ�ѧ����ʽΪ__________________________________��
(3)��ͬѧ��Ϊ��װ���д��Ż�����β�����ȴ�����ƿ�ڣ�Ȼ���ٴ�������ͼ��������Ƶ�����ƿ��β��Ӧ��__________(�a����b��)��ͨ��(����װ����)��
��
(4)��ͬѧ���ɣ�CO�ܷ�ʹ����ʯ��ˮ����ǣ���ˣ��������COͨ��CuO֮ǰ��Ӧ��ͨ�����ʯ��ˮ���ԱȽ��ų�CO�����ʯ��ˮ��Ӧ���ԶԴ��������ۡ�����Ϊ��������Ƿ��б�Ҫ��____________��������________________��
(5)�����ʼʱͨ�����CO��CO2�Ļ�����壬��Ӧ�����Ʋ��ܴﵽʵ��Ŀ�ģ�_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������CuSO4��5H2O����ͭ����Ҫ��������Ź㷺��Ӧ�á�������CuSO4��5H2O��ʵ�����Ʊ�����ͼ��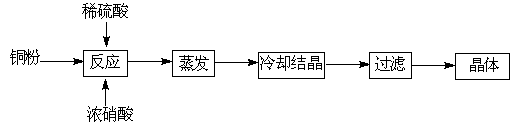
������и��⣺
��1����ͭ�۵�ϡ�����еμ�Ũ���ᣬ����ͭ�۵��ܽ���ܹ۲쵽��ʵ������ �� ��
��2���Ƶõĵ������壨CuSO4��5H2O���п��ܴ��ڵ������� ��д��ѧʽ����
��3�������������ⶨCuSO4��5H2O�ĺ���ʱ���������£�
��ȡ�������� �ڼ�ˮ�ܽ� �ۼ��Ȼ�����Һ���ɳ��� �ܹ��ˣ����ಽ��ʡ�ԣ�
�ڹ���ǰ����Ҫ�����Ƿ������ȫ��������� ��
��. ij�о���ѧϰС����ZRY-1�����ط����Ƕ�12.5������ͭ����(CuSO4��5H2O)�������ط��������¶ȵ����ߣ�����ͭ�������η������з�Ӧ��
a.CuSO4��5H2O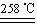 CuSO4��5H2O
CuSO4��5H2O
b.CuSO4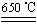 CuO��SO3����2SO3
CuO��SO3����2SO3 2SO2��O2
2SO2��O2
c.4CuO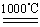 2Cu 2 O��O2��
2Cu 2 O��O2��
��ش��������⣺
��1��ʵ����������ط����Dz�ò�����������Ϊ3.8 g�����ƶϸù��������� (д��ѧʽ)�����Ӧ�����ʵ���֮���� ��
��2�����ط����Ǽ��Ⱦ��������غ�ȫ�����嵼����ͨ������������Һ��ַ�Ӧ�����ó��������ˡ�ϴ�ӡ��������Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
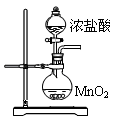
���Ȼ��ף�PCl3����һ����Ҫ���л��ϳɴ�����ʵ���ҳ��ú���������Cl2��ȡPCl3��װ������ͼ��ʾ��
��֪������������Cl2��Ӧ����PCl3�������Cl2��Ӧ����PCl5��PCl3��O2������POCl3(��������)�� POCl3����PCl3��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl��PCl3��POCl3���۷е���±���
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �� | | ��ҺX�к���Na+ |
| �� | | ��ҺX�к���Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���ҳ���MnO2��Ũ���ᷴӦ�Ʊ�Cl2(��Ӧװ����ͼ��ʾ)��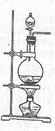
��1���Ʊ�ʵ�鿪ʼʱ���ȼ��װ�������ԣ��������IJ���������__________������ţ���
A������ƿ�м���MnO2��ĩ B������ C������ƿ�м���Ũ����
�Ʊ���Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ�ѷ��������MnO2��ķ�Ӧ����Һ�������Ũ�ȣ�̽��С��ͬѧ���������ʵ�鷽����
������������AgNO3��Һ��Ӧ���������ɵ�AgCl������
�ҷ���������֪��CaCO3(����)��Ӧ������ʣ������������
��������������Zn��Ӧ���������ɵ�H2�����
�̶����������жϺ�ʵ�飺
��2�������������������______________________________________________________��
��3���ҷ�����ʵ�鷢�֣������к���MnCO3��˵��̼�����ˮ�д���__________________,�ⶨ�Ľ���______________________���ƫ����ƫС����ȷ������
���б�����ʵ�飺װ����ͼ��ʾ���г���������ȥ����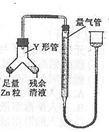
��4��ʹY�ι��еIJ�����Һ��п����Ӧ����ȷ�����ǽ�________ת�Ƶ�______�С�
��5����Ӧ��ϣ�ÿ���1���Ӷ�ȡ����������������������С��ֱ�����䡣 ���������μ�С��ԭ����____________���ų�װ�ú�ʵ�������Ӱ�����أ���
��6��С��������ͬѧ������ɲ�������к͵ζ����ⶨ����Һ�������Ũ�ȣ������辭��������֪����________________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���� Y ��������������Ͽ��Խ�������ʵ�飨�̶�װ���ԣ����������ش��������⣺
��1��ʵ��Ŀ�ģ���֤SO2�������ԡ�����ͷ�ι���Ũ����ֱ���� Y�ܵ�����֧���У���������������������������Ӧ��SO2+2H2S=3S+2H2O������֧�ܽ��洦ʵ������Ϊ ������������ˮ��Ŀ���� ��
��2��ʵ��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ���ɳ�����������SO2ͨ��BaCl2��Һ����������������ͨ����һ�������Ͳ����˰�ɫ�����������£������Ҳ� Y�ܲ�����һ�����壬����������֧��Ӧ���õ�ҩƷ�� �� ��������A�������� ��
��3��ʵ��Ŀ�ģ���þ�Ͻ����������IJⶨ���ٶ�ȡ������������ʱ��������ˮ���е�Һ�������������Һ�棬Ӧ��ȡ�Ĵ�ʩ�� �������Ƶ���þ�Ͻ������Ϊ 0.080g���������г�����Ϊ1.00mL��ĩ����Ϊ 45.80mL��������Ϊ��״��������Ͻ������İٷֺ���Ϊ ����ȷ��0.01%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ͭ��һ��Ӧ�ù㷺�Ļ���ԭ�ϣ�ʵ�����п�ͨ����ͬ;����ȡ����ͭ��Һ�͵���(CuSO4��5H2O)������һ���������£�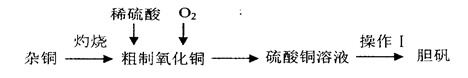
(1)����I����Ϊ__________��___________�����ˡ���ɡ�
(2)��ͭ(�������л���)���պ�IJ��������ͭ��������ͭ��ԭ�������___________(����ĸ����)��
a�����չ����в�������ͭ����ԭ
b�����ղ���֣�ͭδ����ȫ����
c������ͭ�ڼ��ȹ����зֽ�����ͭ
d����������ͭ������������
(3)������ͭ��Ϊ������ͭ�ۣ���ֱ�������м���ϡ�����Fe2(SO4)3��Һ������ͨ����������Ӧ��ȫ�������м������_______________(�ѧʽ����ͬ)������pH��4������_________���������˵�����ͭ��Һ����֪Fe(OH)3��Cu(OH)2��ȫ����ʱ��pH�ֱ�Ϊ3��7��6��4]��
��4)ͨ������ͼ1װ��Ҳ����ȡ����ͭ��Һ(��֪��2NaOH+2NO2��NaNO3+NaNO2+H2O)��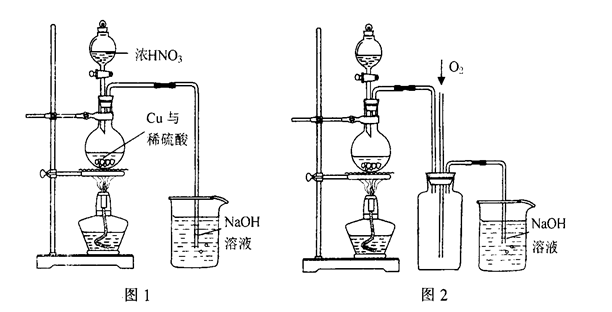
��ƿ�ڷ�����Ӧ�����ӷ���ʽΪ____________________________________________��
ͼ2��ͼ1�ĸĽ�װ�ã����ŵ��Т�__________________________����_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��û���ֳɵ�CO2���巢����������£�����ѡ����ͼ��ʾ����������װ���һ�����ġ����濪���á������ͣ��CO2���巢��װ�á�
��1��Ӧѡ�õ�������________________(������)��
��2����������װ����ȡCO2���壬��ʵ����ֻ��ϡ���ᡢŨ���ᡢˮ����״�����״����ʯ���ȽϺ����ķ�����Ӧѡ�õ�ҩƷ��________ _________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ���Ӧ�Ľ��۲���ȷ����( )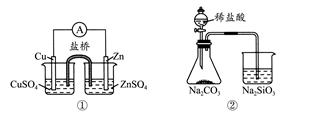
| A���������Zn��Cuԭ��� |
| B������֤���ǽ�����Cl>C>Si |
C����˵����Ӧ2NO2(g)  N2O4(g)����H<0 N2O4(g)����H<0 |
| D���ܰ�ɫ����ΪBaSO4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com