
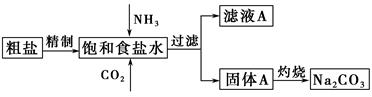


 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������þ���۵���ڽ����� |
| B����������ʵ��ۡ��е��Li��Cs�������ߵ� |
| C������þ��Ӳ��С�ڽ����� |
| D����������Ӳ�ȴ��ڽ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �Ƿ���ȫ�����IJ������������ϲ���Һ�м����μӳ�������Һ
�Ƿ���ȫ�����IJ������������ϲ���Һ�м����μӳ�������Һ| A���٢� | B���ڢ� | C���ڢ� | D���٢� |
�鿴�𰸺ͽ���>>
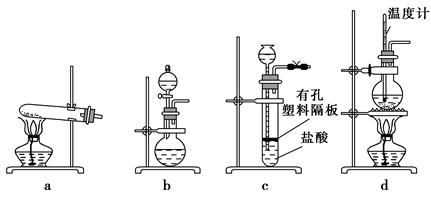
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2��Fe2O3 |
| B��CO��CuO |
| C��H2��Na2CO3 |
| D��CO��Na2CO3 |
�鿴�𰸺ͽ���>>
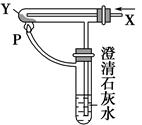
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ձ���һ�����ᷢ�������ӷ�Ӧ�У�2Na��2H2O=2Na����2OH����H2�� |
| B�������ձ����ƾ���Һ���Ͼ��ҷ�Ӧ����ȶ��ԣ�X�ձ��еķ�Ӧƽ��Щ |
| C��Z�ձ���һ�����г������ɣ����������ǵ���ͭ |
| D�������ձ����û�������������ʵ���һ����ͬ |
�鿴�𰸺ͽ���>>
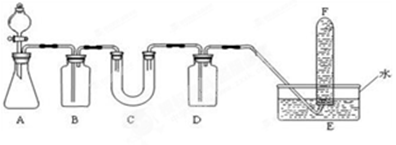
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | �����Լ� | ������Լ���Ŀ�� |
| B | ����NaHCO3��Һ | ��2�֣� |
| C | Na2O2 | ��2�֣� |
| D | NaOH��Һ | ��2�֣� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
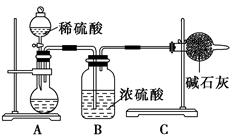
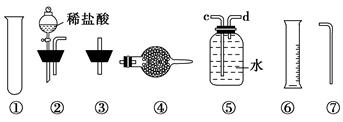
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O | B��Na2O2 |
| C��Na2O��Na2O2 | D��Na2O2��NaO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȣ�����������ų� |
| B���μ����ᣬ����������ų� |
| C������ˮ�μ�ϡ���Ȼ�����Һ�����ް�ɫ�������� |
| D������ˮ�μӳ���ʯ��ˮ�����ް�ɫ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com