����⣺��1��Ҫ��ȥSO
42-��ֻ��ѡBaCl
2��Һ����ѡ��Ba��NO
3��
2���������µ�����NO
3-����ѡ��NaOH��Һ��ȥMg
2+��Fe
3+��Һ�����ѡ��Na
2CO
3��Һ��ȥCa
2+���˴�����ѡ��K
2CO
3��Һ������������µ�K
+������HCl��ȥ������CO
32-��Na
2CO
3��Һ���ܼ���BaCl
2��Һǰ�����������Ba
2+��
�ʴ�Ϊ��BaCl
2��NaOH��Na
2CO
3��
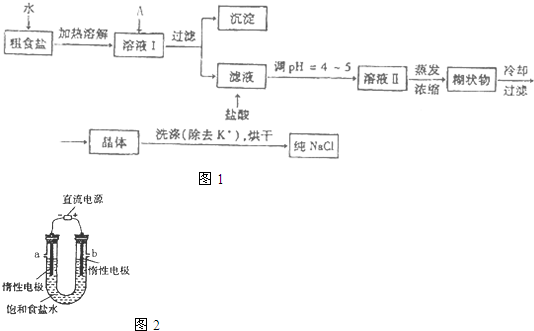
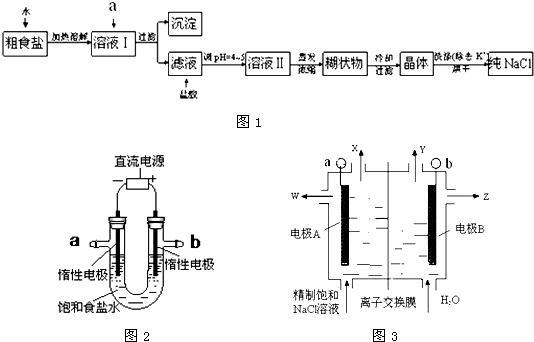
��2������ʵ�����ᴿNaCl������ͼ��ʵ��Ŀ�Ŀ�֪����Ũ����Һ���dz�ȥ��Һ�е�Ca
2+��Mg
2+��Fe
3+��SO
42-���ӵõ��ĺ�״�����������Ҫ��ѧ�ɷ����Ȼ��ƣ�
�ʴ�Ϊ��NaCl��
��3������500mL4.00mol?L
-1NaCl��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������Ҫ��������������ƽ���ձ�����������500ml������ƿ����ͷ�ιܡ�ҩ�ף�ʹ�û���Ҫ������ƽ��500ml������ƿ����ͷ�ιܣ�
�ʴ�Ϊ��������ƽ��500ml������ƿ����ͷ�ιܣ�
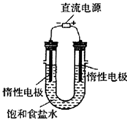
��4����ͼ2��ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H
2O
2NaOH+Cl
2��+H
2����������ˮ����һ�������ܽ⣬������Cl
2�������ɵ�NaOH��ӦNaCl��NaClO��H
2O��ʹ�ò��ֵ�Cl
2�����ģ�����ͬ���������ռ�����Cl
2С��2L��bΪ��Դ����������2Cl
--2e
-=Cl
2����������װ�õ�b���ܷ�ס������һ��ʱ���2NaCl+2H
2O
2NaOH+Cl
2��+H
2���٣�2NaOH+Cl
2=NaCl+NaClO+H
2O�ڣ���+�ڵô�����Һ��һ���ܷ�Ӧ����ʽ��NaCl+H
2O
NaClO+H
2����
�ʴ�Ϊ������������ˮ����һ�������ܽ⣬������ɵ������������ɵ�NaOH�����˷�Ӧ��NaCl+H
2O
NaClO+H
2����
��5��A�������ӽ���Ĥ������Na
+ͨ�������ʱ��������������������NaCl����Ӧ����������NaCl������BΪ����������2H
++2e
-=H
2������A����
B�����ʱ��������������������NaCl��W��ϡ���Ȼ�����Һ����B��ȷ��
C����������OH
-����Na
+�������ƶ������Ʒ�ռ���Һ��Z�ڵ�������C��ȷ��
D�������ӽ���Ĥ������Na
+ͨ�������ʱ��������������������NaCl����Ӧ����������NaCl������AΪ������aΪ��Դ������������2Cl
--2e
-=Cl
2������BΪ������bΪ��Դ����������2H
++2e
-=H
2������D����
��ѡBC��



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�