
ЁОЬтФПЁПАДвЊЧѓЬюПеЃК
(1)3.6gH2OЮяжЪЕФСПЮЊ________molЃЌдМКЌга_______ИідзгЃЛ
(2)вбжЊ1.204ЁС1023ИіXЦјЬхЕФжЪСПЪЧ6.4gЁЃдђXЦјЬхЕФФІЖћжЪСПЪЧ________ЃЛ
(3)жЦБИFe(OH)3НКЬхЕФЛЏбЇЗНГЬЪНЃК________ЃЛ
(4)ЪЕбщЪвЭЈГЃгУMnO2КЭХЈбЮЫсЙВШШжЦШЁCl2ЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃЈMnCl2ЪЧПЩШмадбЮЃЉMnO2+4HCl(ХЈ)![]() MnCl2+Cl2Ёќ+2H2OЃЌИУЗДгІжабѕЛЏМСЪЧ_______ЃЌбѕЛЏВњЮяЪЧ________(ЬюЛЏбЇЪН)ЃЌаДГіЩЯЪіЛЏбЇЗНГЬЪНЖдгІЕФРызгЗНГЬЪН__________ЁЃ
MnCl2+Cl2Ёќ+2H2OЃЌИУЗДгІжабѕЛЏМСЪЧ_______ЃЌбѕЛЏВњЮяЪЧ________(ЬюЛЏбЇЪН)ЃЌаДГіЩЯЪіЛЏбЇЗНГЬЪНЖдгІЕФРызгЗНГЬЪН__________ЁЃ
ЁОД№АИЁП0.2 0.6NAЛђ3.612ЁС1023 32g/mol FeCl3+3H2O![]() Fe(OH)3(НКЬх)+3HCl MnO2 Cl2 MnO2+4H+
Fe(OH)3(НКЬх)+3HCl MnO2 Cl2 MnO2+4H+![]() Mn2++Cl2Ёќ+2H2O
Mn2++Cl2Ёќ+2H2O
ЁОНтЮіЁП
(1)ИљОнn=![]() МЦЫуЮяжЪЕФСПЃЌНсКЯN=nЁЄNAМАH2OжаКЌга3ИідзгМЦЫудзгЪ§ФПЃЛ
МЦЫуЮяжЪЕФСПЃЌНсКЯN=nЁЄNAМАH2OжаКЌга3ИідзгМЦЫудзгЪ§ФПЃЛ
(2)ЯШИљОнN=nЁЄNAМЦЫуЦфЮяжЪЕФСПЃЌШЛКѓРћгУn=![]() МЦЫуФІЖћжЪСПЃЛ
МЦЫуФІЖћжЪСПЃЛ
(3)НЋБЅКЭТШЛЏЬњШмвКЕЮШыЗаЬкЕФеєСѓЫЎжаМгШШжСвКЬхГЪКьКжЩЋЃЌПЩжЦШЁЧтбѕЛЏЬњНКЬхЃЛ
(4)дкИУЗДгІжадЊЫиЛЏКЯМлЩ§ИпЃЌЪЇШЅЕчзгЃЌБЛбѕЛЏЃЌзїЛЙдМСЃЌБЛбѕЛЏЮЊбѕЛЏВњЮяЃЛдЊЫиЛЏКЯМлНЕЕЭЃЌЕУЕНЕчзгЃЌБЛЛЙдЃЌзїбѕЛЏМСЃЌБЛЛЙдЮЊЛЙдВњЮяЃЛИљОнРызгЗНГЬЪНЪщаДддђНЋЗНГЬЪНИФаДЮЊРызгЗНГЬЪНЁЃ
(1)3.6gH2OЮяжЪЕФСПn(H2O)=3.6gЁТ18g/mol=0.2molЃЌИљОнN= nЁЄNAМАH2OжаКЌга3ИідзгПЩжЊ0.2molH2OжаКЌгаЕФдзгЪ§ФПN=0.2molЁСNA/mol=0.2NAЃЛ
(2)1.204ЁС1023ИіXЦјЬхЕФЮяжЪЕФСПn=1.204ЁС1023ЁТ6.02ЁС1023/mol=0.2molЃЌгЩгкЦфжЪСПЪЧ6.4gЃЌдђXЦјЬхЕФФІЖћжЪСПЪЧM=6.4gЁТ0.2mol=32g/molЃЛ
(3)НЋБЅКЭТШЛЏЬњШмвКЕЮШыЗаЬкЕФеєСѓЫЎжаМгШШжСвКЬхГЪКьКжЩЋЃЌПЩжЦШЁЧтбѕЛЏЬњНКЬхЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃКFeCl3+3H2O![]() Fe(OH)3(НКЬх)+3HClЃЛ
Fe(OH)3(НКЬх)+3HClЃЛ
(4)дкИУЗДгІжаЃЌClдЊЫиЛЏКЯМлЩ§ИпЃЌЪЇШЅЕчзгЃЌБЛбѕЛЏЃЌHClзїЛЙдМСЃЌБЛбѕЛЏЕФCl2ЮЊбѕЛЏВњЮяЃЛMnдЊЫиЛЏКЯМлНЕЕЭЃЌЕУЕНЕчзгЃЌБЛЛЙдЃЌMnO2зїбѕЛЏМСЃЌБЛЛЙдЮЊЕФMnCl2ЮЊЛЙдВњЮяЃЛИљОнРызгЗНГЬЪНЪщаДддђЃЌЩЯЪіЛЏбЇЗНГЬЪНЖдгІЕФРызгЗНГЬЪНЮЊЃКMnO2+4H++2Cl-![]() Mn2++Cl2Ёќ+2H2OЁЃ
Mn2++Cl2Ёќ+2H2OЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПбЧЯѕЫсФЦ(NaNO2)ЪЧвЛжжживЊЕФЙЄвЕбЮЃЌвзШмгкЫЎЃЌЮЂШмгкввДМЁЃФГЛЏбЇаЫШЄаЁзщЖдбЧЯѕЫсФЦНјааЖрНЧЖШЬНОПЃК
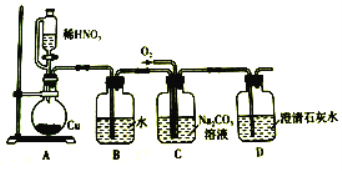
Ђё.бЧЯѕЫсФЦЕФжЦБИЁЃ
ЃЈ1ЃЉзАжУBЕФзїгУЪЧ________________________ЁЃ
ЃЈ2ЃЉDжаГЮЧхЪЏЛвЫЎБфЛызЧЃЌдђCжажЦБИNaNO2ЕФРызгЗНГЬЪНЮЊ______________ЁЃ
Ђђ.ЬНОПбЧЯѕЫсФЦгыСђЫсЗДгІ![]() ЦјЬхВњЮяГЩЗжЁЃ
ЦјЬхВњЮяГЩЗжЁЃ
вбжЊЃКЂйNOЃЋNO2ЃЋ2OHЃ=2NOЃЋH2O
ЂкЦјЬхвКЛЏЕФЮТЖШЃКNO2ЮЊ21 ЁцЃЌNOЮЊЃ152 Ёц

ЃЈ3ЃЉЗДгІЧАгІДђПЊЕЏЛЩМаЃЌЯШЭЈШывЛЖЮЪБМфЕЊЦјЃЌФПЕФЪЧ_______________________ЁЃ
ЃЈ4ЃЉвЧЦїЕФСЌНгЫГађ(АДзѓЁњгвСЌНг)ЃКAЁњCЁњ________ЁЃ
ЃЈ5ЃЉдкЙиБеЕЏЛЩМаЃЌДђПЊЗжвКТЉЖЗЛюШћЃЌЕЮШы70%СђЫсКѓЃЌзАжУAжаВњЩњКьзиЩЋЦјЬхЁЃШєDжаЭЈШыЙ§СПO2ЃЌзАжУBжаЕФЛЏбЇЗНГЬЪНЪЧ_______________________ЁЃ
Ђѓ.ЩшМЦЪЕбщжЄУїЫсадЬѕМўЯТNaNO2ОпгабѕЛЏадЁЃ
ЃЈ6ЃЉЙЉбЁгУЕФЪдМСЃКNaNO2ШмвКЁЂKMnO4ШмвКЁЂFe2(SO4)3ШмвКЁЂKIШмвКЁЂЯЁСђЫсЁЂЕэЗлШмвКЁЂKSCNШмвК___________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖдгкФГаЉРызгЕФМьбщМАНсТлвЛЖЈе§ШЗЕФЪЧ
A. МгШыЯЁбЮЫсВњЩњЮоЩЋЦјЬхЃЌНЋЦјЬхЭЈШыГЮЧхЪЏЛвЫЎжаЃЌШмвКБфЛызЧЃЌвЛЖЈгаCO![]()
B. МгШыТШЛЏБЕШмвКгаАзЩЋГСЕэВњЩњЃЌдйМгбЮЫсЃЌГСЕэВЛЯћЪЇЃЌвЛЖЈгаSO![]()
C. МгШыЧтбѕЛЏФЦШмвКВЂМгШШЃЌВњЩњЕФЦјЬхФмЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЃЌвЛЖЈгаNH![]()
D. МгШыЬМЫсФЦШмвКВњЩњАзЩЋГСЕэЃЌдйМгбЮЫсАзЩЋГСЕэЯћЪЇЃЌвЛЖЈгаBa2ЃЋ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЗДгІНјааЗжРрЪБЃЌМШЪєгкбѕЛЏЛЙдЗДгІгжЪєгкжУЛЛЗДгІЕФЪЧ (ЁЁЁЁ)
A. CH4ЃЋ2O2![]() CO2ЃЋ2H2O B. 2KClO3
CO2ЃЋ2H2O B. 2KClO3![]() 2KClЃЋ3O2Ёќ
2KClЃЋ3O2Ёќ
C. SЃЋO2![]() SO2 D. 8NH3+3Cl2=6NH4Cl+N2Ёќ
SO2 D. 8NH3+3Cl2=6NH4Cl+N2Ёќ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПКЃбѓзЪдДЕФРћгУОпгаЙуРЋЧАОАЁЃ
ЃЈ1ЃЉЮоашОЙ§ЛЏбЇБфЛЏОЭФмДгКЃЫЎжаЛёЕУЕФЮяжЪЪЧ___(ЬюађКХ)
A.Cl2 B.ЕЫЎ C.ЩеМю D.ЪГбЮ
ЃЈ2ЃЉШчЭМЪЧДгКЃЫЎжаЬсШЁУОЕФМђЕЅСїГЬЁЃ
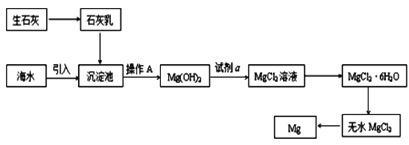
ЂйВйзї AЪЧ___ЁЃ
ЂкКЃЫЎЬсУОЕФЙ§ГЬЃЌЮЊЪВУДвЊНЋКЃЫЎжаЕФТШЛЏУОзЊБфЮЊЧтбѕЛЏУОЃЌдйзЊБфЮЊТШЛЏУОЃП___
ЃЈ3ЃЉРћгУКЃЕзЕФЁАПЩШМБљЁБжЦзїЕФЫсадШМСЯЕчГиЕФзмЗДгІЪНЮЊЃКCH4+2O2=CO2+2H2OЃЌдђИУШМСЯЕчГиИКМЋЕФЕчМЋЗДгІЪНЮЊ____ЁЃ
ЃЈ4ЃЉКЃДјЛвжаИЛКЌвд I- аЮЪНДцдкЕФЕтдЊЫиЃЌЪЕбщЪвЬсШЁ I2ЕФЭООЖШчЭМЫљЪОЃК
![]()
ЂйзЦЩеКЃДјжСЛвН§ЪБЫљгУЕФжївЊвЧЦїЪЧ____ЃЈЬюађКХЃЉЁЃ
a.лслі bЃЎЪдЙм cЃЎеєЗЂУѓ dЃЎЩеБ
ЂкЯђЫсЛЏЕФТЫвКжаМгЙ§бѕЛЏЧтШмвКЃЌаДГіИУЗДгІЕФРызгЗНГЬЪН____ЁЃ
ЃЈ5ЃЉКЃЕзЕФУКОзлКЯРћгУПЊЗЂЕФИБВњЮяCO2ФмЩњВњМзДМШМСЯЃЌЦфЗДгІЕФЗНГЬЪНЮЊЃКCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)ЃЌФГПЦбЇЪЕбщНЋ6molCO2КЭ8molH2ГфШы2LЕФУмБеШнЦїжаЃЌВтЕУH2ЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЭМЪЕЯпЫљЪОЃЌaЃЌbЃЌcЃЌd РЈКХФкЪ§ОнБэЪОзјБъЁЃ
CH3OH(g)+H2O(g)ЃЌФГПЦбЇЪЕбщНЋ6molCO2КЭ8molH2ГфШы2LЕФУмБеШнЦїжаЃЌВтЕУH2ЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЭМЪЕЯпЫљЪОЃЌaЃЌbЃЌcЃЌd РЈКХФкЪ§ОнБэЪОзјБъЁЃ
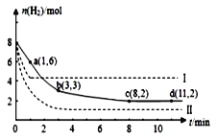
ЂйaЕуе§ЗДгІЫйТЪ___(ЬюЁАДѓгкЁЂЕШгкЛђаЁгкЁБ)aЕуФцЗДгІЫйТЪЁЃ
ЂкЦНКтЪБCO2ЕФЮяжЪЕФСПХЈЖШЪЧ____mol/LЁЃ
ЂлФмЙЛЫЕУїИУЗДгІДяЕНЛЏбЇЦНКтзДЬЌЕФБъжОЪЧ___ЁЃ
AЃЎЕЅЮЛЪБМфФкЯћКФ1molCO2ЃЌЭЌЪБЩњГЩ3molH2 BЃЎЛьКЯЦјЬхЕФУмЖШВЛЫцЪБМфБфЛЏ
CЃЎCH3OHЁЂH2ЕФХЈЖШВЛдйЫцЪБМфБфЛЏ DЃЎCH3OHКЭH2OХЈЖШЯрЕШ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП3 mol AКЭ2.5 mol BЛьКЯгк2 LУмБеШнЦїжаЃЌЗЂЩњЕФЗДгІЃК3A(g)ЃЋB(g)![]() xC(g)ЃЋ2D(g) 5minКѓЗДгІДяЕНЦНКтЃЌШнЦїФкбЙЧПБфаЁЃЌВтЕУDЕФЦНОљЗДгІЫйТЪЮЊ0.1 mol/(LЁЄmin)ЃЌЯТСаНсТлВЛе§ШЗЕФЪЧ
xC(g)ЃЋ2D(g) 5minКѓЗДгІДяЕНЦНКтЃЌШнЦїФкбЙЧПБфаЁЃЌВтЕУDЕФЦНОљЗДгІЫйТЪЮЊ0.1 mol/(LЁЄmin)ЃЌЯТСаНсТлВЛе§ШЗЕФЪЧ
A. AЕФЦНОљЗДгІЫйТЪЮЊ0.15mol/(LЁЄmin)
B. ЦНКтЪБЃЌAЕФзЊЛЏТЪЮЊ20ЃЅ
C. ЦНКтЪБЃЌCЕФХЈЖШЮЊ 0.25 mol/L
D. ШнЦїФкЕФЦ№ЪМбЙЧПКЭЦНКтбЙЧПжЎБШЮЊ11:10
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЭКЭХЈСђЫсПЩвддкМгШШЬѕМўЯТЗЂЩњШчЯТЗДгІ(ЗДгІЗНГЬЪНвбХфЦН)ЃКCuЃЋ2H2SO4(ХЈ)![]() CuSO4ЃЋAЁќЃЋ2H2OЪдЭЈЙ§МЦЫуКЭЭЦРэЭъГЩЯТУцЕФЮЪЬтЃК
CuSO4ЃЋAЁќЃЋ2H2OЪдЭЈЙ§МЦЫуКЭЭЦРэЭъГЩЯТУцЕФЮЪЬтЃК
(1)AЮяжЪПЩвдЕМжТЫсгъЕФаЮГЩЁЃдђAгІИУЪєгк______________(гУзжФИДњКХЬюаД)
aЃЎЫс bЃЎМю cЃЎбЮ dЃЎЫсадбѕЛЏЮя eЃЎМюадбѕЛЏЮя
(2)SO2гыO2ЕФЛьКЯЦјЬхжаЃЌбѕдЊЫиЕФжЪСПЗжЪ§ЮЊ70%ЃЌдђSO2гыO2ЕФЮяжЪЕФСПжЎБШЪЧ__________ЃЌетжжЛьКЯЦјЬхЕФУмЖШЪЧЭЌЮТЭЌбЙЯТбѕЦјУмЖШЕФ_____БЖЁЃ
(3)вЛЖЈСПЕФЭЦЌгы100mL 18mol/L ЕФХЈH2SO4ГфЗжЗДгІЃЌШчЙћИУЗДгІЙ§ГЬжазЊвЦСЫ0.2molЕчзгЃЌЩњГЩЕФCuSO4ЕФЮяжЪЕФСПЮЊ_________molЃЌ
(4)НЋЗДгІКѓЫљЕУЕНЕФCuSO4ШмвКгызуСПBa(OH)2ШмвКГфЗжЗДгІЕФРызгЗНГЬЪНЪЧ_____________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЪЧХфжЦ100mL2molЁЄL-1ЕФNaOHЕФШмвКЙ§ГЬЪОвтЭМЃК

ЃЈ1ЃЉВНжшЃЈ3ЃЉЃЈ4ЃЉЃЈ5ЃЉЃЈ6ЃЉЖМЩцМАЭЌвЛвЧЦїЃЌДЫвЧЦїЕФУћГЦЮЊ___ЁЃ
ЃЈ2ЃЉХфжЦДЫШмвКЪБЃЌашГЦШЁNaOHЙЬЬхЕФжЪСПЮЊ___gЁЃ
ЃЈ3ЃЉВНжшЃЈ6ЃЉВйзїКѓЃЌЗЂЯжвКУцЯТНЕЃЌетЪБ___ЃЈЬюЁАашвЊЁБЛђЁАВЛашвЊЁБЃЉдйМгЫЎжСПЬЖШЯпЁЃ
ЃЈ4ЃЉЪЕбщЪБЃЌШчЙћУЛгаВНжшЃЈ4ЃЉЕФВйзїЃЌЫљЕУШмвКЕФЮяжЪЕФСПХЈЖШНЋ___ЃЛЖЈШнЪБИЉЪгПЬЖШЛсЕМжТХЈЖШ___(ЬюЁАЦЋИпЁБЁАВЛБфЁБЛђЁАЦЋЕЭ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгаШ§жжПЩШмадЮяжЪAЁЂBЁЂCЃЌЦфжаAЁЂBЪєгкбЮЃЌCЪєгкМюЃЌЫќУЧШмгкЫЎКѓЕчРыВњЩњЕФЫљгаРызгШчЯТБэЫљЪОЃК
бєРызг | NaЃЋ HЃЋ Ba2ЃЋ |
вѕРызг | OHЃ CO32Ѓ SO42Ѓ |
ЧыИљОнЯТСаа№ЪіЛиД№ЮЪЬтЃК
ЃЈ1ЃЉCЕФЛЏбЇЪНЮЊ___ЁЃ
ЃЈ2ЃЉAШмвКгыBШмвКЗДгІПЩЩњГЩЦјЬхЃЌдђИУЗДгІЕФРызгЗНГЬЪНЮЊ___ЁЃ
ЃЈ3ЃЉAЁЂBШмвКгыCШмвКЗДгІПЩЗжБ№ЩњГЩАзЩЋГСЕэDКЭEЃЌЦфжаDПЩШмгкЯЁЯѕЫсЁЃ
ЂйBЕФЛЏбЇЪНЮЊ___ЃЌМјБ№ШмвКжаBЕФвѕРызгЕФЪдМСЮЊ___ЁЃ
ЂкDШмгкЯЁЯѕЫсЕФРызгЗНГЬЪНЮЊ___ЁЃ
ЂлDгыEЕФЛьКЯЮяagЃЌМгШызуСПбЮЫсЃЌЭъШЋЗДгІЩњГЩЕФЦјЬхдкБъзМзДПіЯТЬхЛ§ЮЊVLЃЌдђEдкЛьКЯЮяжаЕФжЪСПЗжЪ§ЕФБэДяЪНЮЊ___ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com