


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
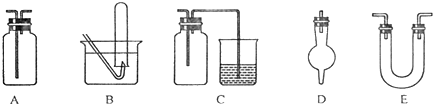
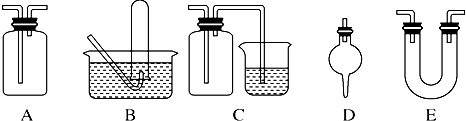
 ��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ��������Ҫ�������м���Һ�����壩��
��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ��������Ҫ�������м���Һ�����壩��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���֣���1��������ͭ������ᾧˮ�����IJⶨʵ���У�������������Ҫ���� �Ρ�
��2���к��Ȳⶨ��ʵ���У��õ��IJ����������ձ����¶ȼơ� �� ��
(3)��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ(������Ҫ�������м���Һ������)��

��ش��������⣺
�����������ﰱ����װ����_______________(����ĸ)��
�ڼ��������ռ��������������ռ�һ�����������װ����_______________(����ĸ)��
����ʵ�����Ʊ�������ʵ���У����Գ�ȥ�������Ȼ�������������װ����________________ (����ĸ)��
����������ϩ����ˮ��Ӧ�ƶ��������ʵ��װ����___________________(����ĸ)��
����Cװ���У������ձ��ڵ�����������Һ����β��������������ƿ��������___________________��
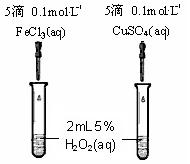 ��4��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�顣
��4��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�顣
�ٿ�ͨ���۲� �����Եĵó����ۣ�
����Aͬѧ�����CuSO4��ΪCuCl2��Ϊ������������ ��
��������Aͬѧ�ĸĽ�����������Ϊ��������θ� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����������һ�и�����һ��ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
��15�֣�
��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ(������Ҫ�������м���Һ������)��
��ش��������⣺
�����������ﰱ����װ����___________(����ĸ)��
�Ƽ��������ռ��������������ռ�һ�����������װ����____________(����ĸ)��
��������������Ӧʵ���У����������������ͷ�Ӧװ��֮���Գ�ȥ�������Ȼ�������������װ����____________(����ĸ)��
����������ϩ����ˮ��Ӧ�ƶ��������ʵ��װ����_____________(����ĸ)��
������Cװ���������������ձ�������������Һ��Ӧ��ʵ�飬�����й��ƿ��������________________________________________________________________��
��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�顣
�ٿ�ͨ���۲� ���������ԱȽϵó�
���ۡ�
����ͬѧ�����CuSO4��ΪCuCl2��Ϊ�������������� ��
����Ϊ���������θĽ���
�q
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com