
ЗжЮіФГжжУКЦјЕФЬхЛ§зщГЩШчЯТЃКH250%ЁЂCH430%ЁЂCO10%ЁЂN26%ЁЂCO24%ЁЃ
вбжЊЃКH2ЃЈgЃЉ+![]() O2ЃЈgЃЉ=H2O(1);ЁїH=Ѓ285.8kJ?molЃ1
O2ЃЈgЃЉ=H2O(1);ЁїH=Ѓ285.8kJ?molЃ1
CO(g)+ ![]() O2(g)=CO2(g); ЁїH=Ѓ282.6kJ?molЃ1
O2(g)=CO2(g); ЁїH=Ѓ282.6kJ?molЃ1
CH4(g)+2O2(g)=CO2(g)+2H2O(1); Ёї=Ѓ890.3kJ?molЃ1
дђдкБъзМзДПіЯТЃЌ224LИУжжУКЦјШМЩеЪБЗХГіЕФШШСПЮЊ ЃЈ ЃЉ
AЃЎ1461.7kJ BЃЎ4382.5kJ CЃЎ4665.1kJ DЃЎ5811.5kJ

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| 1 |
| 2 |
| 1 |
| 2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
вбжЊЃКH2ЃЈgЃЉ+![]() O2ЃЈgЃЉ====H2O(l);ІЄH=-285.8 kJЁЄmol-1
O2ЃЈgЃЉ====H2O(l);ІЄH=-285.8 kJЁЄmol-1
CO(g)+![]() O2(g)====CO2(g);ІЄH=-282.6 kJЁЄmol-1
O2(g)====CO2(g);ІЄH=-282.6 kJЁЄmol-1
CH4(g)+2O2(g)====CO2(g)+2H2O(l);ІЄH=-890.3 kJЁЄmol-1
дђдкБъзМзДПіЯТЃЌ224 LИУжжУКЦјШМЩеЪБЗХГіЕФШШСПЮЊ( )
A.1461.7 kJ B.4382.5 kJ C.4665.1 kJ D.5811.5 kJ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК

Ђђ.вбжЊXЁЂYЁЂZЁЂWЪЧЖЬжмЦкжаЕФЫФжжЗЧН№ЪєдЊЫиЃЌЫќУЧЕФдзгађЪ§вРДЮдіДѓЁЃXдЊЫидзгаЮГЩЕФРызгОЭЪЧвЛИіжЪзгЃЌZЁЂWдкдЊЫижмЦкБэжаДІгкЯрСкЕФЮЛжУЃЌЫќУЧЕФЕЅжЪдкГЃЮТЯТОљЮЊЮоЩЋЦјЬхЃЌYдзгЕФзюЭтВуЕчзгЪ§ЪЧФкВуЕчзгЪ§ЕФ2БЖЁЃ
ЃЈ1ЃЉНігЩXЁЂZЁЂWШ§жждЊЫизщГЩЕФФГжжбЮЪЧвЛжжЫйаЇЗЪСЯЃЌЕЋГЄЦкЪЉгУЛсЪЙЭСШРЫсЛЏЃЌгаЙиЕФРызгЗНГЬЪНЮЊЃК____________ЁЃ
ЃЈ2ЃЉетЫФжждЊЫиПЩзщГЩдзгИіЪ§БШЮЊ5ЁУ1ЁУ1ЁУ3ЕФЛЏКЯЮяЃЈАДXЁЂYЁЂZЁЂWЕФЫГађЃЉЃЌИУЛЏКЯЮяЕФЫЎШмвКгызуСПЕФNaOHШмвКЗДгІЕФРызгЗНГЬЪНЮЊ____________ЁЃ
ЃЈ3ЃЉНЋ9 g YЕЅжЪдкзуСПWЕЅжЪжаШМЩеЁЃЫљЕУЦјЬхЭЈШы1 L 1 molЁЄL-1ЕФNaOHШмвКжаЃЌЭъШЋЮќЪеКѓЃЌШмвКжаЕФРызгХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
Ђё.ЪЕбщБэУїЃЌCuOБЛH2ЛЙдЪБвВгаCu2OЩњГЩЁЃНЋвЛЖЈСПЕФH2ЛКТ§ЭЈЙ§зЦШШЕФCuOЗлФЉЃЌЕУЕНЙЬЬхЛьКЯЮяЧвЛьКЯЮяжаm(Cu)ЁУm(O)=8ЁУaЁЃЕБaгаВЛЭЌЕФШЁжЕЪБЃЌЙЬЬхЛьКЯЮяГЩЗжВЛЭЌЁЃЧыЗжЮіaЕФШЁжЕЗЖЮЇКЭЙЬЬхЛьКЯЮяГЩЗжЕФЙиЯЕВЂЬюШыЯТБэЃЈВЛвЛЖЈЬюТњЃЌБэИёВЛЙЛвВПЩздаадіМгЃЉЁЃ
aЕФШЁжЕЗЖЮЇ | ЗДгІКѓЙЬЬхЕФГЩЗжЃЈгУЛЏбЇЪНБэЪОЃЉ |
|
|
|
|
|
|
Ђђ.ЛЦЭПѓЕФжївЊГЩЗжXЪЧгЩCuЁЂFeЁЂSШ§жждЊЫизщГЩЕФИДбЮЃЌЦфжаCuЁЂFeСНжждЊЫиЕФжЪСПБШЮЊ8ЁУ7ЃЛНЋm g XЗлФЉШЋВПШмгк200 mLЕФХЈHNO3ЃЌЗДгІКѓЕФШмвКМгЫЎЯЁЪЭжС2.12 LЪБВтЕУЦфpHЮЊ0ЃЛНЋЯЁЪЭКѓЕФШмвКЗжЮЊСНЕШЗнЃЌЯђЦфжавЛЗнШмвКжаЕЮМг6.05 molЁЄL-1ЕФNaOHШмвКЃЌЯђСэвЛЗнШмвКжаЕЮМг0.600 molЁЄL-1 Ba(NO3)2ШмвКЃЌСНШмвКжаОљЩњГЩГСЕэЃЌЧвГСЕэЕФжЪСПЫцЫљМгШмвКЕФЬхЛ§БфЛЏШчЯТЭМЫљЪОЃК
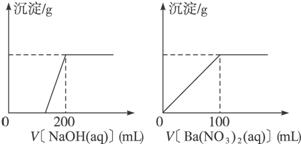
ЃЈ1ЃЉЧыЭЈЙ§МЦЫуШЗЖЈmЕФжЕЃЛ
ЃЈ2ЃЉXЕФФІЖћжЪСПЮЊ368 gЁЄmol-1,ЧыШЗЖЈXЕФЛЏбЇЪН_______________
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com