
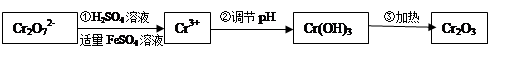
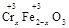 ��y
��y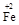 O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������
O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������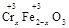 ��y
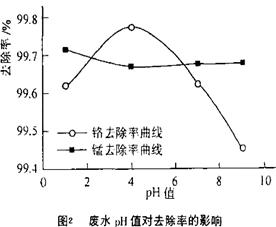
��y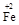 O����ѧʽ�� ��
O����ѧʽ�� ��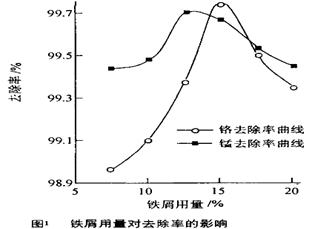
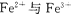
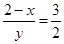 ��3x��6��3x��2y��8�����x��0.5��y��1�����Ը���������Ļ�ѧʽ��Cr0.5Fe1.5O3��FeO��
��3x��6��3x��2y��8�����x��0.5��y��1�����Ը���������Ļ�ѧʽ��Cr0.5Fe1.5O3��FeO��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
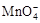 ����C2O42?�����ӷ���ʽ����������
����C2O42?�����ӷ���ʽ����������| ����KMnO4��Һ�Ĵ��� | KMnO4��Һ��ɫ��ȥ�����ʱ�� |
| �ȵ����1�� | 60 s |
| ��ɫ���ٵ����2�� | 15 s |
| ��ɫ���ٵ����3�� | 3 s |
| ��ɫ���ٵ����4�� | 1 s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
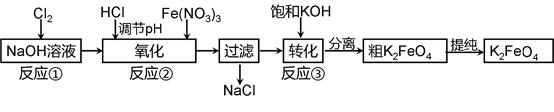
 4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��
4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��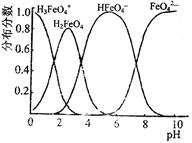
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��NaHSO4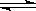 Na++H++SO42һ Na++H++SO42һ | B��NaHCO3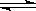 Na++ HCO3һ Na++ HCO3һ |
C��H2S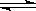 2H++S2������ 2H++S2������ | D��Mg(OH)2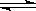 Mg2++2OH- Mg2++2OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ca(OH)2��Һ��ͨ�������CO2 | B����NaAlO2��Һ�е������������ |
| C�����Ȼ�����Һ�еμӹ����İ�ˮ | D����Al2(SO4)3��Һ�е��������NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
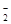 ��Na+��Cl-����Na2O2��HCl��Al2O3�����ʵ���֮����Ϊ�� ��
��Na+��Cl-����Na2O2��HCl��Al2O3�����ʵ���֮����Ϊ�� ��| A��4��6��1 | B��8��6��1 | C��3��3��1 | D��4��6��3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com