
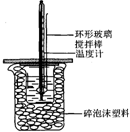
 50mL 1.0mol•L-1�����50mL 1.1mol•L-1����������Һ��ͼ��ʾװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺
50mL 1.0mol•L-1�����50mL 1.1mol•L-1����������Һ��ͼ��ʾװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�/t2/�� | �²t2-t1��/�� |
| 1 | 25.0 | 32.6 | |
| 2 | 25.1 | 31.8 | |
| 3 | 25.1 | 31.9 |
���� ��1���������ȼƵĹ������жϸ�װ�õĴ�С�ձ�����������ĭ���ϵ����ã�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5���������������ᷴӦ���������ᱵ���������ɳ����Ĺ����л��������仯��
��6�����ж����η�Ӧ�¶Ȳ����Ч�ԣ�Ȼ�����ƽ��ֵ�����ݹ�ʽQ=cm��T���������0.05mol��ˮ�ų��������������к��ȵĸ�������к��ȣ�
��7��a��ʵ��װ�ñ��¡�����Ч�������ɢʧ�ϴ�
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ��
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�ϴ�
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨHCl��Һ���¶ȣ�HCl��Һ����ʼ�¶�ƫ�ߣ�
��� �⣺��1���������ȼƵĹ����ʵ��ijɰܹؼ����жϸ�װ�õĴ�С�ձ�����������ĭ���ϵ������DZ��¡����ȣ���������ɢʧ��
�ʴ�Ϊ�����¡����ȣ���������ɢʧ��
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹���������ͭ�ĵ���Ч�����ڻ��β����������
�ʴ�Ϊ�����ܣ��������ȣ���������ɢʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫ�ͣ�
��4����Ӧ�ų����������������Լ�������Ķ����йأ�50mL 1.0mol•L-1�����50mL 1.1mol•L-1����������Һ���з�Ӧ������ˮ����Ϊ0.050mol������60mL 1.0mol•L-1�����50mL 1.1mol•L-1����������Һ���з�Ӧ������0.055molH2O������ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�
�ʴ�Ϊ�����ӣ���������0.055molH2O����ǰ��ֻ������0.050molH2O�����䣻�к�����ָ�������кͷ�Ӧ����1molH2Oʱ���ų��������������������أ�
��5��������Ba��OH��2��Һ��Ӧ��������ˮ�⣬��������BaSO4�������÷�Ӧ�е������Ȼ�Ӱ�췴Ӧ�ķ�Ӧ�ȣ����Բ�����Ba��OH��2��Һ���������NaOH��Һ��������к��ȣ�
�ʴ�Ϊ�����ܣ���Ϊ������Ba��OH��2��Һ��Ӧ����BaSO4�����������Ȼ�Ӱ�췴Ӧ�ķ�Ӧ�ȣ�
��6��3�η�Ӧǰ���¶Ȳ�ֱ�Ϊ��7.6�桢6.7�桢6.8�棬��һ����ȥ��ƽ��ֵΪ6.75�棬50mL1.0mol/L�����50mL1.1mol/L����������Һ��������m=100mL��1g/mL=100g��c=4.18J/��g•�棩�����빫ʽQ=cm��T������0.05mol��ˮ�ų�����Q=4.184J/��g•�棩��100g��6.75��=2.8224kJ��������0.05mol��ˮ�ų�����2.8224kJ����������1mol��ˮ�ų�����Ϊ2.8224kJ��20=56.5kJ������ʵ���õ��к��ȡ�H=-56.5kJ/mol��
�ʴ�Ϊ��-56.5kJ/mol��
��7��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b����
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���c��ȷ��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨHCl��Һ���¶ȣ�HCl��Һ����ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����d��ȷ��
�ʴ�Ϊ��acd��
���� ���⿼���к��Ȳⶨԭ������Ӧ�ȵļ��㣬��Ŀ�Ѷȴ�ע�������к��ȵĸ����Լ��ⶨ��Ӧ�ȵ��������⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ճ�������ʹ�õ�Ӳ�Ҷ��Ǵ��𡢰�������� | |
| B�� | �ܻ����������к�����������ʳƷ���Ӽ� | |
| C�� | SO2������Ư��ֽ�ţ����Ư���ճ�������ʳ����ͷ | |
| D�� | ��ҩ�����֡���ʵ��û�п�ѧ���ݵģ�ֻҪ��Ч������Ҳ����ν |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������ʵ���Ũ�ȵ�Na2SO3��Һ��NaHSO3��Һ��Ϻ���Һ�����ԣ���c��HSO3-����c��SO32-�� | |
| B�� | 0.1mol•L-1��CH3COONa��Һ20mL��0.1mol•L-1��ϡ����10mL��Ϻ���Һ�����ԣ�c��CH3COO-����c ��Cl-����c��H+����c��CH3COOH�� | |
| C�� | c��NH4+��Ũ����ͬ��NH4Cl����NH4��2SO4����NH4��2CO3��Һ�У����ʵ����ʵ���Ũ�ȣ�c[��NH4��2SO4]��c[��NH4��2CO3]��c��NH4Cl�� | |
| D�� | 0.1mol•L-1NaNO3��Һ��0.1mol•L-1CH3COOH��Һ�����������ϣ�c��H+��=c��OH-��+c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����壮
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����壮| �� �� | �� | �� |
| ��Ӧ��Ͷ���� | 1molCO2��3molH2 | a molCO2��b molH2��c molCH3OH��g����c molH2O��g�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ϡ���ᷴӦ | B�� | ����������ұ���� | ||
| C�� | �����백ˮ��Ӧ | D�� | �ܵ�ú��ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 17.9mol•L-1��34.7% | B�� | 20.4mol•L-1��33.8% | ||
| C�� | 17.9mol•L-1��33.8% | D�� | 20.4mol•L-1��34.7% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
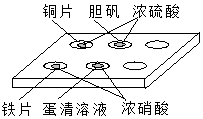 ����ͼ��ʾ��ʵ���У��Ե�ΰ��ϵ�����������ȷ���ǣ�������
����ͼ��ʾ��ʵ���У��Ե�ΰ��ϵ�����������ȷ���ǣ�������| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com